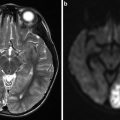
Fig. 1
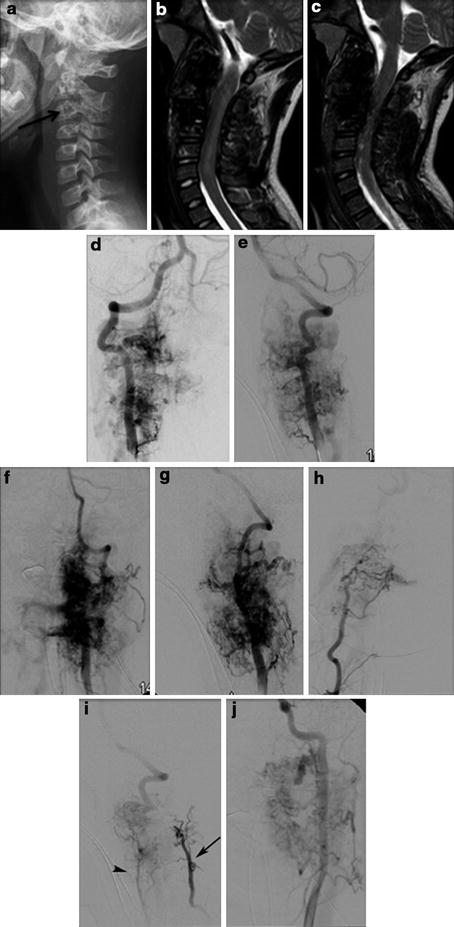
Middle-aged man with subacute onset of progressive paraparesis with bladder and bowel involvement had hyperintense signal in the MRI T2-WI (arrowhead) with enlarged perimedullary veins (White Arrow) (a). T4 intercostal artery angiogram reveals supply to the dural arteriovenous fistula (b–e). Super selective angiogram shows the exact location of the fistula with multiple feeders (Black Arrow). The fistula is treated by n-BCA embolization with obliteration of the proximal segment of the vein just distal to the fistula (Black Arrow in f). Control angiogram reveals total obliteration of the dural arteriovenous fistula (g). Three months later MRI reveals significant improvement in signal changes in the cord (arrowhead in h) and total obliteration of the dorsal perimedullary veins
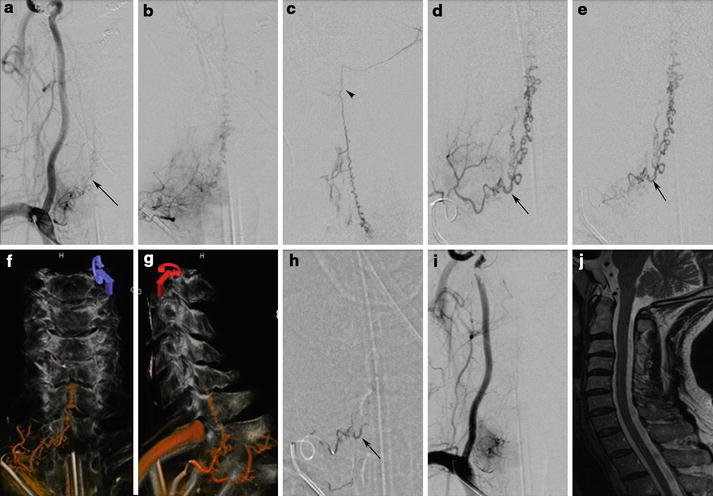
Fig. 2
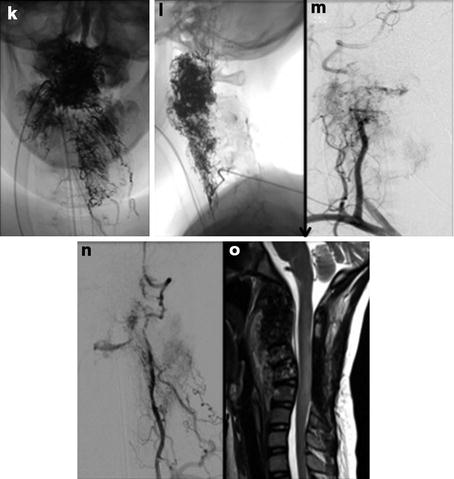
Right subclavian angiogram shows the cervical dural arteriovenous fistula (arrow in a) supplied by a dural branch from thyrocervical trunk. Super selective angiograms (b–e) reveal the anatomic architecture of the dural fistula. Arrowhead in (c) shows the perimedullary venous drainage that drains intracranially. Arrow in (d, e) represents the proximal segment of the vein just distal to the fistula that needs to be targeted for embolization. Intra-procedural DYNA CT with contrast injection shows (f, g) the anatomic location of the fistula in relation to the bone. The embolization performed with n-BCA penetration into the proximal portion of the vein just distal to the fistula (h) (Arrow). Control angiogram reveals total obliteration of the fistula (i). Posttreatment MRI reveals complete obliteration of the fistula as evidenced by absence of enlarged perimedullary veins (J)
Management
The natural history of untreated spinal dAVFs is generally thought to be poor with progressive neurological deficit. The spinal dAVF may be treated either by surgery or endovascular obliteration.
In most patients, the fistula may be obliterated by super selective catheterization of the feeding radiculomeningeal artery and obliteration of the fistula using liquid embolic agents like n-butyl cyanoacrylate (n-BCA) and Onyx. The embolic agent must pass the nidus and reach and occlude the proximal segment of the draining vein to prevent subsequent intradural collateral filling of the fistula (Figs. 1 and 2). At the end of embolization, the collateral circulation of the dural involved region must be examined for the presence of natural arterial collaterals, ipsilateral above and below the level of the fistula as well contralateral at the same level of the spinal dAVF. The success rate of endovascular therapy varies from 25 % to 75 % and recurrence is not uncommon. Up to 80 % of patients have clinical improvement of various degrees to clinical stabilization following embolization. Complications of embolization include inadvertent closure of the spinal radiculomedullary artery mostly due to failure to recognize. Inadvertent deposition of liquid embolic material into the perimedullary veins beyond the nidal-venous junction can result in thrombosis of the spinal cord veins. In cases where endovascular embolization is not possible, placement of a metal coil in the major feeding artery can facilitate intraoperative fluoroscopic localization of the fistula [23].
Surgical ligation is performed by coagulation and division of the intradural radicular vein that receives blood from the shunt resulting in visible change in venous turgor and color of the arterialized venous plexus. This is more invasive but a relatively simple and effective intervention except for sacral fistulas. The key to successful treatment is precise localization of the fistula.
Complete occlusion of the fistula by either method stops disease progression, but only two-thirds of the patients have improvement in motor symptoms, while about one-third show improvement of their sensory disturbances. In most patients, impotence and sphincter disturbances do not recover. In patients with no improvement within 4–6 weeks after treatment, repeat MRI imaging should be done. MRI changes may or may not completely reverse; if persistent flow voids are visualized, it warrants another angiographic evaluation.
Type II: Intramedullary Arteriovenous Malformation
Intramedullary arteriovenous malformation (AVM) consists of malformation within the substance of the spinal cord supplied by the radiculomedullary and radiculopial arteries and drained by the pial veins. They include spinal cord glomerular AVMs with compact or diffuse nidus and AV fistulas that may be intramedullary or perimedullary in location.
Epidemiology
Intramedullary AVM is the second most common spinal vascular lesion, accounting for about 20 % of spinal vascular lesions. There is slight male predominance and they commonly come to clinical attention in the second and third decade of life [24]. They are most commonly located in the thoracolumbar region followed by the cervical region. Syndromes associated with intramedullary spinal AVMs include neurofibromatosis and the Rendu-Osler-Weber, Klippel-Trenaunay-Weber, and Parkes Weber syndromes [25].
Pathophysiology
Glomerular spinal AVMs (nidal AVMs) are more frequent of spinal cord AVMs. They are characterized by a vascular nidus located intramedullary and have multiple feeding arteries derived from both the anterior and posterior spinal arterial systems. They drain into the dilated spinal cord veins. They are distributed throughout the spinal cord: about 30 % in the cervical region but most commonly found in the thoracolumbar region comprising of 70 % that includes conus medullaris AVMs [24], a special category of spinal cord AVMs that are attributed to an abnormality during neurulation and may be associated with a tethered cord [26]. Conus medullaris AVMs possess multiple arterial feeders and are extensive.
Clinical Presentation
Spinal AVMs may present acutely in most cases. Hemorrhage is the most common presentation and more so in children than adults [15, 27]. It may be either subarachnoid or intraparenchymal hemorrhage. The typical syndrome of spinal hemorrhage gives rise to severe pain, which frequently starts in the back and rapidly spreads to the rest of the back and nuchal area and to the legs. In severe hemorrhage in the cervical region, the blood can spread to the intracranial cavity and may cause headaches and disturbances of consciousness. Rehemorrhage is more common with spinal AVMs than in the brain. It constitutes about 10 % in the first month and 40 % in the first year after the event [28]. In adults, the correlation between angioarchitecture and hemorrhage is found as suggested by arterial, nidal aneurysm, pial venous ectasia and congestion, and associated arteriovenous fistulas.
Progressive symptoms are mostly nonspecific with features of hypesthesia, paresthesia, weakness, bladder and bowel dysfunction, impotence, and diffuse back and muscle pain. These symptoms can have a stuttering course sudden worsening followed by some improvements over time. The causes for deterioration are multiple. Among the well-accepted causes are intraspinal hemorrhage, thrombosis within the AVM, arachnoiditis, and increased venous pressure. AVMs may become symptomatic by venous congestion alone, in the absence of intraparenchymal or subarachnoid hemorrhage [29].
Location of the AVM does have influence on the clinical presentation: cervical lesions typically present as progressive sensory deficits, whereas thoracic AVMs most commonly present as hemorrhage (hematomyelia or subarachnoid hemorrhage) and often show progressive myelopathy. Lesions of the conus and filum terminale manifest as progressive myelopathy or acute nonhemorrhagic paraplegia.
Aggravating factors including maneuvers that increase intra-abdominal or intrathoracic pressure such as the Valsalva maneuver and bending often increase medullary venous pressure. Pregnancy is known to aggravate symptomatology by increasing the intra-abdominal pressure, increased intravascular volume, hormonal changes, and added stress on the venous circulation during delivery. Trauma as a precipitating factor for symptoms is not well supported.
Apart from neurologic symptoms, spinal deformities, and complications of the spinal cord, dysfunctions including urinary tract infections, respiratory infections, and decubitus ulcerations are taken into consideration in determining the morbidity and final outcome of these patients.
Imaging
MRI
On MRI the glomerular spinal AVMs show focal dilatation of the cord in the region of the lesion with conglomerate dilated, perimedullary, and intramedullary vessels. There may be an area of low signal intensity around the nidus on T1-WI and T2-WI corresponding to hemosiderin deposition. The enlarged vessels appear as flow voids on T2-WI imaging and as mixed hyperintense to hypointense tubular structures on T1-weighted (T1-WI) imaging (depending on their flow velocity and direction). The edema due to venous congestion appears as cord swelling with intramedullary hyperintensity on T2-WI [29]. Intraparenchymal hemorrhages demonstrate variable signal intensity on T1-WI and T2-WI based on the age of hemorrhage.
MRI plays a significant role in defining the location of the AVM in relation to the spinal cord and dura and can also distinguish between low-flow and high-flow forms of AVMs. As the small arterial feeders and draining veins escape detection on non-contrast MRI, contrast-enhanced fast MR angiography is capable of detecting the main arterial feeder of glomerular-type AVMs. Contrast-enhanced MRI is useful to detect subtle venous dilatations [30, 31].
Angiography
Catheter angiography is the gold standard evaluation for spinal cord AVMs [24]. A complete angiogram characterizes all feeding vessels, nidal configuration, flow characteristics, intranidal aneurysms, and venous drainage with or without venous ectasias. It is essential for treatment plan. Selective catheterization and injection of numerous arteries are essential to fully characterize a spinal AVM. The feeding vessels may arise from respective or distant radiculomedullary arteries supplying the anterior or posterior spinal arteries. The sources as far afield as the occipital, ascending pharyngeal, vertebral, ascending and deep cervical, supreme intercostal, intercostal, lumbar, and lateral and median sacral arteries [28] (Figs. 3 and 4).
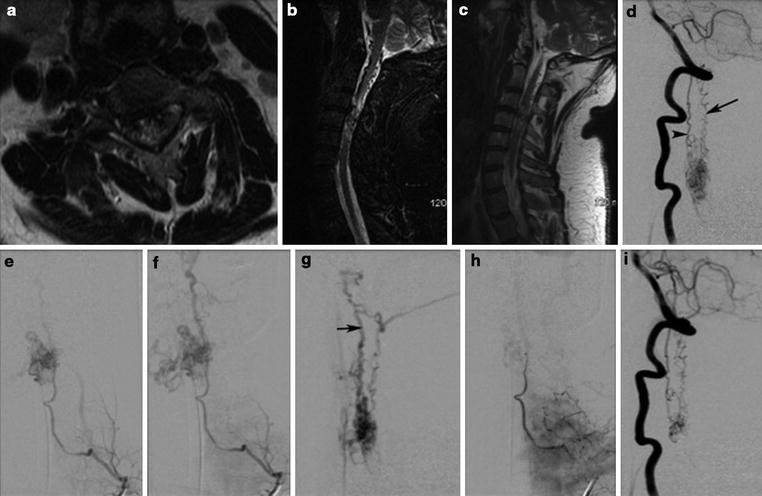
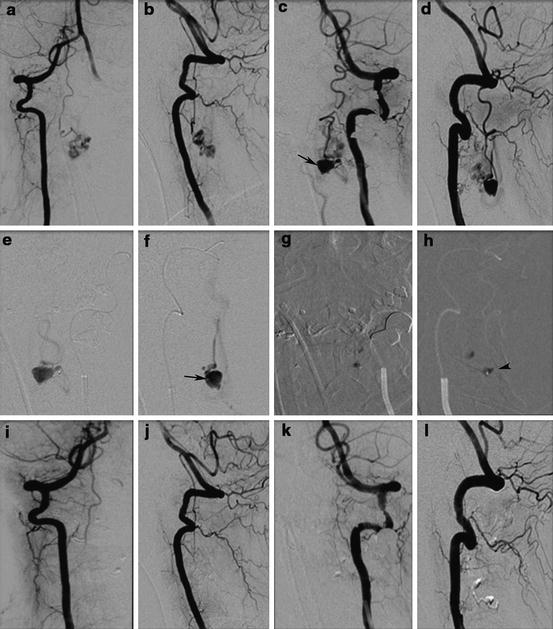

Fig. 3
Intramedullary AVM with perimedullary venous drainage cranially (a–c) has gliotic changes in the spinal cord. DSA reveals supply from the left vertebral artery through anterior and posterolateral spinal arteries (d) and dominant supply from the left radiculomedullary artery arising from the thyrocervical trunk supplying the anterior spinal artery (e, f). The AVM has a dominant cranial venous drainage into the perimedullary venous system (arrow in g). Selective partial embolization of the AVM was performed and control angiogram reveals significant reduction in the supply to the malformation from the thyrocervical trunk with preservation of the anterior spinal axis (h). There is residual supply to the malformation from the left vertebral artery (i)

Fig. 4
Young patient with cervical intramedullary AVM with fistula presented with cervical subarachnoid hemorrhage due to perimedullary large venous ectasia. DSA examination shows anterior spinal artery from the right vertebral artery supplying part of the AVM (a, b). The dominant supply to the AVM is from the posterolateral spinal artery arising from the left vertebral artery (c, d). Note the venous ectasia (arrow in c). Super selective angiogram reveals the fistulous component of the AVM with venous ectasia (e, f). Embolization of the AVM performed with n-BCA, which is deposited in the AVM and ectatic venous sac (g, h). Post-embolization control angiogram reveals complete obliteration of the AVM (i–l)
Management
The natural history of spinal AVMs is unknown. In general, symptomatic spinal AVMs presenting with hemorrhage or progressive neurologic deficit warrant treatment to ameliorate symptoms and improve the outcome. Due to the eloquence of spinal cord and complex nature of spinal AVM architecture, any modality of treatment procedure has a relatively higher complication risk. Management strategies have to be individualized based on the available expertise.
The therapy of choice for all spinal cord AVMs is endovascular embolization. Embolization is performed after careful analysis of the AVM by selective spinal angiography and by using an embolic agent appropriate to the specific angioarchitecture. Proximal arterial occlusion will not obliterate the fistula, because of collateral recanalization from the radiculopial network. Obliteration of proximal venous segment should be the target of embolization. In most cases partial embolization is achieved with complete obliteration rates ranging from 25 % to 30 % [28, 32]. Partial obliteration may be an adjunct to surgery; in general, partial embolization improves the outcome, with reduced rehemorrhage risk and improved prognosis in most patients. Transient and permanent complication rates following embolization range from 10.6 % to 14 % [28, 32] (Figs. 3 and 4).
Surgical management requires appropriate case selection. Surgically accessible lesions with compact nidus can be completely resected with a cure rate of up to 94 % of cases [33].
Radiosurgery may be helpful in selected patients as preliminary reports are encouraging. However, it carries a risk of collateral acute and chronic radiation-induced injury to the surrounding spinal cord [34].
Type III: Juvenile Arteriovenous Malformation
Type III lesions aka spinal arteriovenous metameric syndrome (SAMS) are complex AVMs involving structures derived from two or more embryonic layers. These AVMs involve the spinal cord, bony structures, paraspinal musculature, subcutaneous tissue, and skin in the same segment. The portion of the nidus involving the spinal cord typically has neural tissue within its interstices. Cobb syndrome is by characterized skin, bone, and spinal cord involvement aka cutaneous vertebral medullary angiomatosis.
Epidemiology
Type III lesions constitute about 6 % of all spinal vascular malformations. They are equally located in the cervical, thoracic, and lumbar or conus medullaris regions. There is a 2:1 male to female ratio. They come to clinical attention in childhood and early adulthood. They may be multiple in as high as 19 % of patients. They may be associated with Klippel-Trenaunay and Parkes Weber syndromes [28].
Pathophysiology
Type III lesions are AVMs that involve derivatives of all embryonic layers and consist of extensive vascular malformation embedded within the tissue. The segmented mesodermal origin of the endothelial cells establishes the topographic link between these segmentally related targets. The spinal cord AVM is metameric if the myelomere involved corresponds to that of the nerve involved. The extraspinal and limb lesions are usually high-flow arteriovenous fistulas supplied by the segmental arteries like the intercostal or lumbar arteries and corresponding segmental arteries. The cutaneous lesions are fistulas of capillary or arterial type with high-flow characteristics.
Clinical Features
The clinical presentation may be due to spinal cord, paraspinal, limb, or cutaneous lesions. The typical presentation is in children or young adults with pain and/or myelopathy [28]. Spinal cord involvement may cause progressive myeloradiculopathy due to venous congestion, compression due to enlarged medullary veins, and spinal hemorrhage. The paraspinal vascular malformations can present with steal syndrome due to high-flow AVF. The cutaneous vascular malformations are readily apparent and often present with a bruit. The comorbid conditions such as spinal deformities such as kyphosis and scoliosis along with complications common to spinal cord dysfunction such as urinary tract infections, respiratory infections, and decubitus ulcerations must be taken into consideration in determining the morbidity and final outcome of these patients.
Imaging
Multimodality imaging is the key in complete analysis of these types of malformations. MRI is essential to look for the anatomic extent of the disease, the cord edema, and the intradural venous engorgement. Catheter angiography assessment can be difficult as only a part of the lesion can be seen with injection of contrast into any particular artery (Fig. 5).