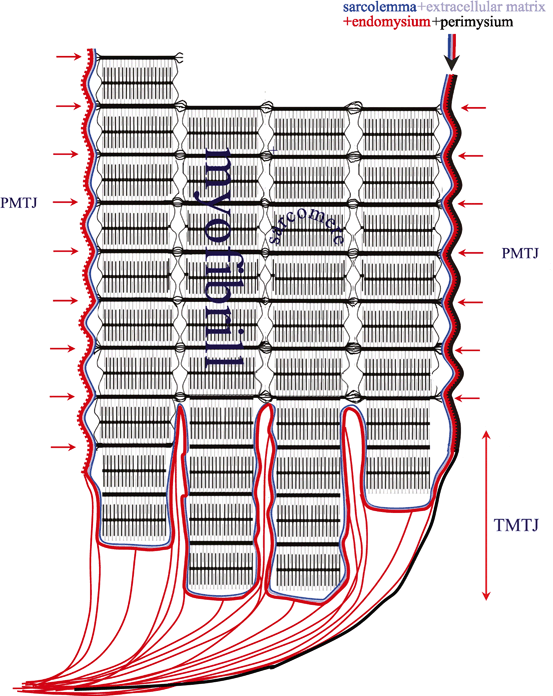
Fig. 13.1
A detailed scheme of a most simple muscle structure with both tendons—semipennate/superficial. Based on a figure from [2]
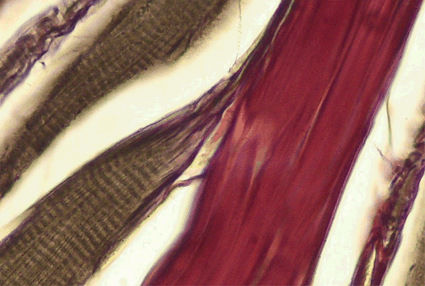
Fig. 13.3
Longitudinal section of a rat soleus muscle, Gomori stain. Red—tendinous system, brown—myofiber. In this section, the terminal myotendinous junction with its tendinous inserts between myofibrils are very well depicted. Based on a figure from [2]
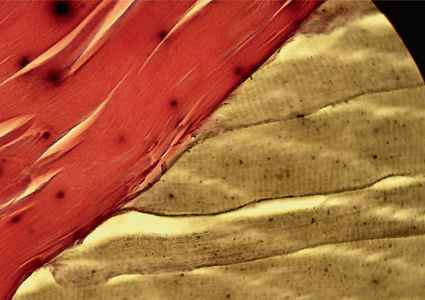
Fig. 13.4
Longitudinal section of a rat soleus muscle, Gomori stain. Red—tendinous system, brown—myofiber. In this section, endo-/perimysial “coat” of the myofiber is very well depicted. Based on a figure from [2]
At the terminal myotendinous junction, tendinous collagen fibrils are inserted into deep recesses formed by myocyte processes, allowing the tension generated by centrally located myofibrils to be transmitted to the collagen fibrils of the terminal myotendinous junction (Fig. 13.2). Sarcomeres attach to endomysium (the tendinous system) at every Z membrane level along the whole length of the myofiber—forming parietal myotendinous junctions (PMTJ) (Fig. 13.2). Human muscles have 500 PMTJ every 1 mm. It is the main force transmission device of the myofiber and of the muscle. At the beginning/end of a myofiber, its myofibrils are separated from each other by endomysium and form terminal myotendinous junction (TMTJ; Fig. 13.2). This is a very important proprioceptive zone as Golgi apparatus as well as Vatter-Paccini and Ruffini corpuscules are largely located here [3]. This complex architecture reduces the tensile stress exerted on the tendons during muscle contraction. It is wrongly understood that the terminal myotendinous junction is the weakest link of a muscle as a whole. In fact, it is supported by both superficial endomysium (Fig. 13.3) and, additionally, by tendinous fibers arising from the TMTJ (Fig. 13.4). What is thought to be a “myotendinous junction” tear is a tendinous tear with frequent involvement of endomysium and perimysium including the level of the TMTJ and PMTJ too. We must remember that 1 mm of the tendon myofiber has already attached to endomysium at 500 levels; close but quite away in terms of myofiber built. At the muscle belly level, tendons may distribute their fibers on one side—these are called semipennate (Fig. 13.1), or on all sides—pennate (Fig. 13.5). This means that semipennate tendon is located on the surface of the muscle and pennate tendon is located within a muscle belly. Many muscles have tendons which run superficially (semipennate) and at some level become pennate—“dive” inside the muscle belly (Fig. 13.5). This may be wrongly interpreted as a tendon end when, in fact, the tendon disappears from the muscle surface [2].
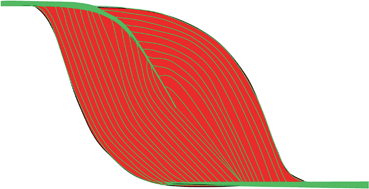

Fig. 13.5
A scheme of a muscle with proximal semipennate and then pennate tendon, distal semipennate tendon. Red—muscle belly, green—tendons and EP. By permission from [4]. EP endomysium and perimysium
Tendons respond to repetitive overload beyond the physiological threshold with either inflammation of their sheath or tearing with possible degeneration of scars of their body or both. Tendon or ligament tears are followed by a repair process which may differ in quality and duration. However, tendon damage may even occur from stresses within the physiological limits as frequent cumulative microtrauma may not allow enough time for repair. Frank inflammatory lesions and granulation tissues are infrequent and are mostly associated with tendon ruptures or other associated chronic inflammatory conditions. The presence of inflamed synovial or fatty tissue (paratenon, bursas, sheaths, and their fatty tissues) may disturb the scar healing process.
Tendon healing occurs in three overlapping phases. Initially, in the inflammatory phase, the erythrocytes and inflammatory cells (particularly neutrophils) enter the site in the first 24 h. This is followed by the proliferative phase which begins after 5 days. Synthesis of type III collagen peaks during this stage and lasts for few weeks. After about 6 weeks, the remodeling phase starts with decreased cellularity as well as reduced collagen synthesis.
Classically, in many cases, tendons with intratendinous lesions detected on US or magnetic resonance imaging (MRI) are not painful. However, pain may be present in patients with tendinopathy, which is usually attributed to inflammation. Pain may originate from a composite of both mechanical and biochemical factors. Anti-inflammatory treatment is usually helpful in such cases and, clinically, it is justified by pain and edema.
Sonographic Findings
Tendons and Ligaments
Tendons and ligaments are those structures that tend to tear first in acute or chronic injuries. The normal fibrillar pattern of tendons and ligaments appears as several long bright echoes surrounded by hypoechoic/anechoic matter. Tendons and ligamentous tears can be stratified into three grades:
Grade I—deformation of a tendon without injury to the collagen fibers. Such deformation may elongate the tendon by 4–6 %. The only visible sonographic feature of the injury is mild edema which makes the fibrillar pattern look a bit darker compared to contralateral side which should be used for measurement reference.
Grade II—some fibers completely torn, some with grade I, whereas some other fibers remain normal. In its early stages (first week or two), a tear appears as hypo-/anechoic focal lesion (reflecting fiber tear and blood metabolites) within the tendon/ligament. At 2–3 weeks, the scar is forming and initially does not have much structure. At 4–6 weeks, the scar tissue is well structured and this scar is called microfibrillar as it is built of finer collagen fibers than the original.
Grade III—a complete tear of the tendon or ligament. Depending on the tendon/ligament and the site, the stumps and separating hematoma/fluid can be seen. Later stumps tend to form a scar between the two torn ends unless they are retracted.
In general, any injury of the tendon/ligament collagen fibers whether in its early stages, i.e., grade I (edematic) or in more severe conditions (grade II or III), is usually associated with unwinding of the torn sites and twisted structures, thus gaining volume. Therefore, it is expected that every tear is most likely associated with thickening of the tendon/ligament structure. That is the least specific feature of a tear.
In the following section, the most common specific tendon injuries are discussed in further detail.
The Shoulder
Rotator cuff injury and inflammation of the subdeltoid bursa are one of the most common causes of shoulder pain. Rotator cuff consists of the tendons of subscapularis, supraspinatus, infraspinatus as well as teres minor muscles in addition to the capsulo-ligamentous complex of the shoulder joint. The strongest part of the capsulo-ligamentous complex is a superior complex and antero-inferior complex. Superior complex [5, 6] is located under both supraspinatus and infraspinatus tendons and consists of superior gleno-humeral ligament, coraco-humeral ligament, and superior–posterior gleno-humeral ligament. The thickness of the complex varies and is of similar thickness to the tendinous part of the cuff [7, 8] (Figs. 13.6 and 13.7). It must be stressed here that the supraspinatus and infraspinatus tendons do not form two layers. The two layers that we see in US (and MRI) are tendinous and capsulo-ligamentous ones [4].
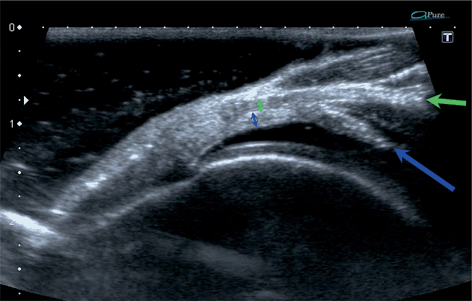
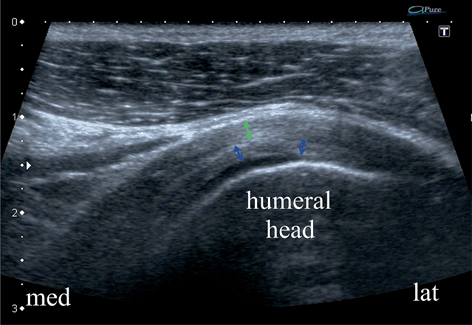

Fig. 13.6
Longitudinal scan of the supraspinatus zone of the normal rotator cuff fresh specimen in a water bath. Tendinous (green arrows) layer, previously bluntly, separated from capsulo-ligamentous layer (blue arrows) on the length of approx. 10 mm

Fig. 13.7
Longitudinal scan of the infraspinatus zone of the normal rotator cuff. Tendinous layer—green arrows, capsulo-ligamentous layer—blue arrows
The antero-inferior complex consists of the medial and inferior gleno-humeral ligaments connected distally by a transversely oriented fasciculus obliquus [6]. This zone also features two layers comparable in thickness. The width of capsulo-ligamentous and tendinous insertion width varies from front to back and fits between 3.5 and 9.1 mm for a capsulo-ligamentous layer, and 3.5–9.7 mm for a tendinous one [8]. When there is diffuse fibrosis, layers cannot be distinguished; however, it can be predicted which layer is damaged, particularly in view of the finding that superior and antero-inferior complex forms at least 1/3–1/2 of the rotator cuff thickness and, in places, reaches 2/3.
Rotator Cuff
The most common type of the rotator cuff injury is tearing, whether full thickness or partial (Figs. 13.8, 13.9, 13.10, and 13.11). Common tear location is enthesis. Regardless of the initial factor, which may be inflammatory or mechanical, it may lead to imbalance between the endurance of the enthesal cortex and the load on either tendon or ligament. Enthesis site is commonly known as “enthesis zone”. Enthesis zone is the enthesis ± 10 mm.
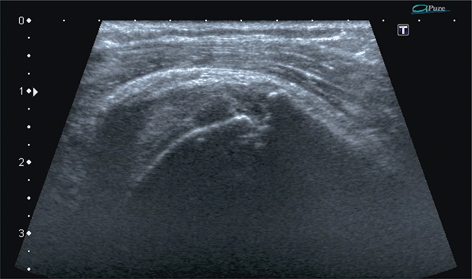
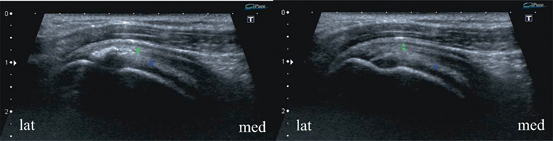
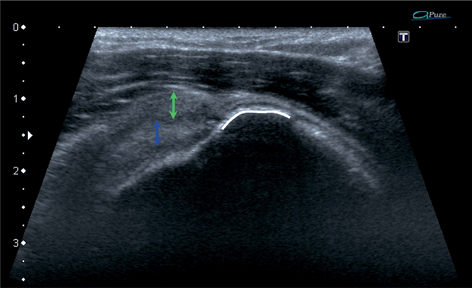
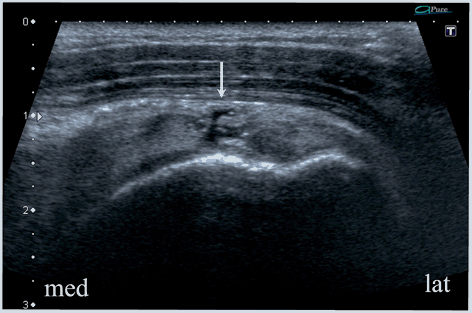

Fig. 13.8
Supraspinatus zone of the rotator cuff, longitudinal scan. Enthesopathy, a scar-filled erosion of the enthesis of the tendinous layer—a chronic tear with bone destruction. Note quite some fibrosis within the subdeltoid–subacromial bursa indicating chronic inflammation or fibrosis directly due to healing process within the tendon

Fig. 13.9
Supraspinatus zone of the rotator cuff, longitudinal scans of two adjacent layers. Enthesopathy with scar mineralization mainly within tendinous layer (left) and on the surface of the superior complex (right). Such mineral cavities within a scar may become quite large, mainly those following tears right at the border of insertion of the tendinous and capsulo-ligamentous complex where shearing forces are generated

Fig. 13.10
Supraspinatus zone of the rotator cuff, longitudinal scan. A total tear of the supraspinatus tendon (green arrow) with preserved continuity of the capsulo-ligamentous layer (blue arrow) allowing little retraction of the tendon. White line is the enthesis from which the tendon was torn off

Fig. 13.11
Supraspinatus zone of the rotator cuff, longitudinal scan. A total tear of the supraspinatus tendon and superior complex. Arrow shows fluid separating the stumps. Little retraction of the torn structures indicates a small tear
There are three main ways how the enthesis zone tears [7]:
1.
Without injury to the bone
2.
With erosive and cystic destruction of the bone
3.
With mineralization or even ossification of the enthesis cartilage and tendinous/ligamentous scar.
There may also be a mixed type.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree