Fig. 1
Approximate vascular territories of the cerebral arteries and their branches as well as the posterior fossa vessels. There is considerable variability in these territories particularly in the basal ganglia, brainstem, and posterior fossa
At the base of the brain, branches of the internal carotid arteries and the vertebrobasilar trunk anastomose to form an arterial polygon known as the circle of Willis [3]. The circle of Willis is formed by two terminal ICAs, two proximal or horizontal (A1) segments of the ACA, the AComm, two PComms, two proximal or horizontal (P1) segments of the PCAs, and the top of the basilar artery (BA). The MCAs are not considered part of the circle of Willis. The normal circle of Willis is depicted diagrammatically in Fig. 2.
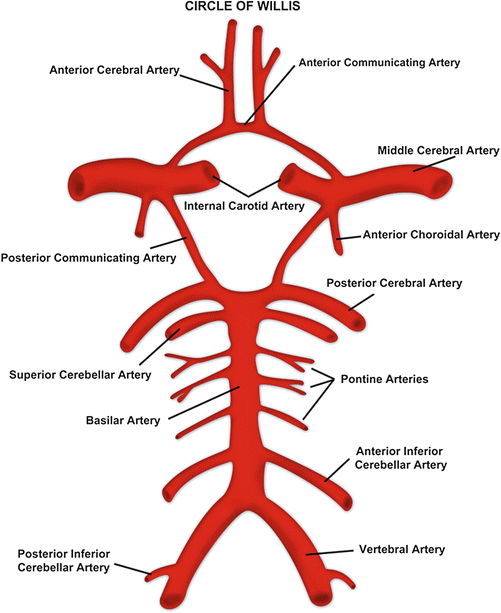

Fig. 2
Anatomic diagram of the normal circle of Willis
The following sections discuss the normal anatomy and vascular distributions of the anterior and posterior circulations as seen on CT and MRI.
Anterior Circulation
Intradural Internal Carotid Artery
The intracranial ICA is divided into six segments: petrous (C2) segment, lacerum (C3) segment, cavernous (C4), clinoid (C5) segment, ophthalmic (C6) segment, and communicating (C7) segment [4]. The ophthalmic (C6) segment is the first ICA segment that lies within the subarachnoid space and gives off two important branches: the ophthalmic artery which supplies the retina and the optic nerve [4] and the superior hypophyseal artery which supplies the anterior pituitary lobe, the infundibular stalk, and the optic chiasm [5].
The communicating (C7) segment extends from the PComm origin to the ICA terminus. This segment has two important branches: the PComm and the anterior choroidal artery.
Posterior Communicating Artery
The PComm joins the anterior and posterior circulation. It extends from the ICA to the junction of the P1 and P2 segments of the PCA. Perforating arteries arise from the posterior PComm and supply the thalamus and hypothalamus [4].
The tuberothalamic artery or polar artery arises from the caudal part of the PComm, which is close to the PCA, or from the border between the caudal and middle third of the PComm [6, 7]. It is absent in approximately one-third of the normal population and is considered to have an anatomically complementary relationship with the paramedian artery originating from the P1 segment [7]. The tuberothalamic artery supplies the ventral section of the thalamus including the anterior thalamic nuclei (i.e., anteromedial, anteroventral, anterodorsal nuclei), ventral anterior nucleus, reticular nucleus, rostral portion of the ventrolateral nucleus, ventral pole of the medial dorsal nucleus, mammillothalamic tract, ventral amygdalofugal pathway, and ventral portion of the internal medullary lamina [6].
Infarction in the territory of the tuberothalamic artery results in severe neuropsychological deficits [7]. A characteristic feature of tuberothalamic artery infarctions is the impairment of recent memory, temporal disorientation, and new learning, which is more prominent with left-sided lesions, while right-sided lesions result in visual memory deficits, hemispatial neglect, and visual spatial processing deficits [7, 6]. Language disorders characterized by anomia, impaired fluency, semantic and phonemic paraphasic errors, and impaired comprehension are seen in left-sided lesions. The “amnestic syndrome” is associated with a disconnection between the anterior thalamic nuclei and hippocampal formation, caused by the disruption of the mammillothalamic tract, as well as a disconnection between the amygdala and anterior nuclei by disruption of the amygdalothalamic projections passing through the internal medullary lamina [8].
Patients with infarctions confined to the ventral anterior nucleus, anterior nuclei, paramedian nuclei, mammillothalamic tract, and internal medullary lamina manifest differently from those with the previously described larger tuberothalamic infarctions. In these patients, there is severe impairment of memory and new learning with relative sparing of language [6]. Palipsychism is a phenomenon associated with anterior thalamic infarcts in which patients demonstrate perseveration which leads to overlap of sequential cognitive processes [9].
Anterior Choroidal Artery
The anterior choroidal artery (AChA) arises from the posterior wall of the ICA distal to the origin of the PComm (although it occasionally arises from the MCA) and courses laterally in the suprasellar cistern to enter the choroidal fissure of the temporal horn of the lateral ventricle [3, 4].
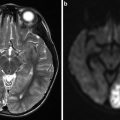
Although the vascular territory of the AChA shows large variations, the most consistently documented area involves the amygdala, the lateral geniculate body, posterior two-thirds of the posterior limb of the internal capsule, most of the globus pallidus, the origins of the optic radiations, the middle one-third of the cerebral peduncles, and the lateral thalamic border [3, 4, 10, 11]. The AChA territory is reciprocal with those of the posterolateral and posteromedial choroidal arteries that arise from the PCA [4]. Figure 3 depicts an infarct in the vascular territory supplied by the AChA.
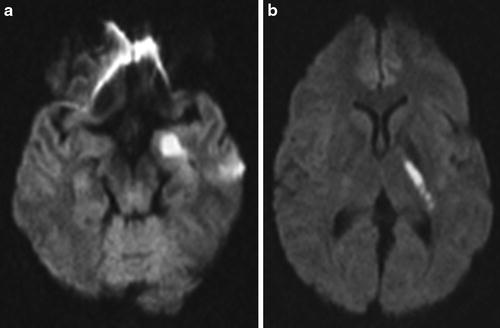

Fig. 3
Thirty-six-year-old female with right hemiparesis and word-finding difficulties. Diffusion-weighted MRI (DWI) obtained 2 h after onset of symptoms demonstrated restricted diffusion in the left mesial temporal lobe (a) and in the left posterior limb of the internal capsule (b) consistent with acute infarction in the left anterior choroidal artery distribution
Interruption of blood flow from the AChA can result in complete or partial manifestation of the AChA syndrome which includes the triad of hemiplegia (due to involvement of pyramidal tracts in the posterior limb of the internal capsule), hemisensory loss (due to involvement of the posterolateral nucleus of the thalamus), and homonymous hemianopia (due to involvement of the lateral geniculate body) [10, 12, 13]. Many AChA territory infarcts present as lacunar syndromes [13].
Normal Variants of the ICA
Fetal origin of the posterior cerebral artery occurs when the caliber of the PComm may be the same as or larger than that of the ipsilateral P1 segment of the posterior cerebral artery [14]. This variant has clinical significance since simultaneous infarcts in the vascular territories of both the anterior and posterior circulation can occur from ICA emboli in the presence of fetal circulation [15].
A “hyperplastic” AChA is defined as a normal cerebrovascular variant in which the temporal-occipital branches of the posterior cerebral artery arise from the AChA [14]. The clinical significance of this variant is the increased occurrence of intracranial aneurysm formation [16].
In a 4-mm embryo, there are sites of anastomosis between the paired dorsal aortic arches that later form the internal carotid arteries and the paired longitudinal neural arteries that later form the vertebrobasilar system [17]. With the exception of the PComm, all other primitive arterial connections regress when the definite circulation develops. Failure of these vessels to regress results in persistent carotid-vertebrobasilar anastomoses. From cephalad to caudal, these are the persistent trigeminal artery, persistent otic artery, persistent hypoglossal artery, and proatlantal intersegmental artery [14, 17].
The persistent trigeminal artery (PTA) is seen in 0.1–0.2 % of patients and is the most common of the carotid-vertebrobasilar anastomoses [3]. Salas et al. classify the PTA into a medial sphenoid variation which has an intrasellar or trans-hypophyseal course and a lateral petrosal variation in which the artery courses with the sensory roots of the trigeminal nerve and exits the Meckel cave below the petroclinoid ligament [18]. Saltzman classifies the PTA into three types: Type I is defined when the PTA supplies the distal basilar artery, the proximal basilar artery is typically hypoplastic, and the ipsilateral PComm is absent, Type II is defined when the PTA fills the anterior superior cerebellar arteries only and the posterior cerebral arteries are supplied by the PComms, and Type III demonstrates a PTA that unites with a remnant of the primitive paired longitudinal neural artery and supplies an ipsilateral cerebellar artery (usually the anterior-inferior cerebellar artery) [19]. Clinically, these anomalies may be responsible for ischemia and trigeminal neuralgia [20].
The persistent hypoglossal artery originates from the ICA at the levels of the C1 through C3 vertebral bodies and courses through the hypoglossal canal to anastomose with the basilar artery [3]. This variant is the second most common carotid-vertebrobasilar anastomosis and has been reported to result in glossopharyngeal neuralgia and hypoglossal nerve paralysis [3, 14].
The proatlantal intersegmental artery arises from the internal carotid artery (Type I) or the external carotid artery (Type II) at the levels of the C2 through C4 vertebral bodies and joins the suboccipital vertebral artery before coursing through the foramen magnum [3, 4].
The persistent otic artery is the least common of the carotid-vertebrobasilar anastomoses. The actual existence of this variant is controversial, and it may represent overlapping vascular territories rather than persistence of an embryonic vessel [4, 14].
Occlusions of the ICA may have clinical manifestations related to embolism or low flow (discussed in border zone infarctions) [15]. Episodes of transient monocular blindness due to embolization to the retinal circulation are typical. An ICA occlusion may be asymptomatic in the presence of a competent circle of Willis. When a thrombus propagates up to the top of ICA and occludes both the MCA and the ACA, the occlusion is often called a “T” occlusion [15]. It carries a very poor prognosis unless complete recanalization is achieved very early [21–23].
Anterior Cerebral Artery
The ACA is the smaller terminal branch of the supraclinoid ICA. The ACA supplies the anterior two-thirds of the medial surface of the cerebral hemisphere extending from the frontal pole to the parieto-occipital sulcus; this region includes the motor and sensory cortical areas for the pelvis and the lower extremities. The ACA vascular territory includes the anterior four-fifths of the corpus callosum, the anterior limb of the internal capsule, the anterior-inferior head of the caudate nucleus, and approximately 1 in. of the frontal and parietal cortex of the cerebral convexity adjacent to the interhemispheric fissure [3, 4]. Figure 4 demonstrates an acute infarct with hemorrhagic conversion in the expected vascular distribution of the ACA. The ACA has three segments: horizontal (A1), vertical (A2), and callosal (A3) segments.
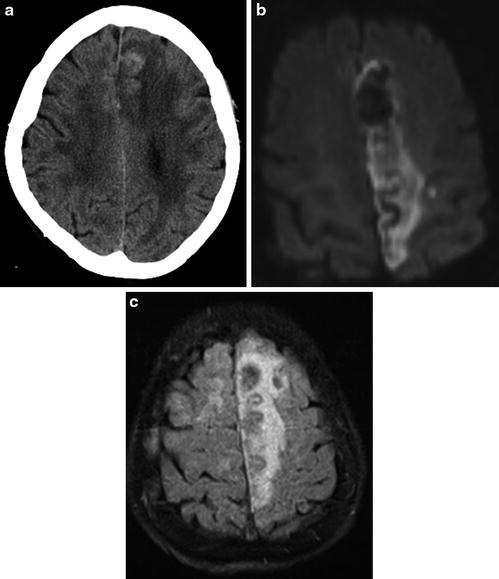

Fig. 4
Sixty-year-old male with weakness of the right shoulder, leg, and foot. Non-contrast-enhanced CT of the brain (a) obtained 6 h after onset of symptoms demonstrates hypoattenuation with superimposed hyperdensity consistent with hemorrhagic conversion of acute infarct in the left anterior cerebral artery distribution. Diffusion-weighted (b) and FLAIR (c) images obtained 1 h later in the same patient show the full extent of acute left anterior cerebral artery infarct
Horizontal (A1) Segment
The horizontal/A1 segment extends from its origin to the midline where it communicates with the contralateral ACA by the AComm. The ACA gives rise to the medial lenticulostriate arteries which supply the medial basal ganglia and the anterior limb of the internal capsule [3, 4] (Fig. 1). The AComm has perforating arteries that supply the anterior hypothalamus, optic chiasm, genu of the corpus callosum, cingulate gyrus, and pillars of the fornix.
Vertical (A2) Segment
Callosal (A3) Segment
The callosal/A3 segment curves around the corpus callosal genu and divides into the pericallosal and callosomarginal arteries. The pericallosal artery courses over the superior surface of the corpus callosum in the pericallosal cistern and gives off many small branches to the corpus callosum [3]. The callosomarginal artery courses within the cingulate sulcus over the cingulate gyrus [3]. The cortical branches of the pericallosal and callosal marginal arteries supply the medial portions of the frontal lobes, superior medial portions of the parietal lobes, and the anterior corpus callosum [24]. Infarctions of these vessels result in hemiparesis and hemianesthesia of the contralateral leg due to involvement of the medial precentral and postcentral gyri, respectively.
Recurrent Artery of Heubner
The recurrent artery of Heubner arises from the proximal A2 or the distal A1 segment [25, 26] and supplies the anterior part of the caudate nucleus, the anterior third of the putamen, the tip of the outer segment of the globus pallidus, and the inferior anterior limb of the internal capsule [25, 26]. Hemiparesis with faciobrachial predominance has been attributed to occlusions within this artery [26]. Figure 5 depicts an acute infarct in the vascular territory of the recurrent artery of Heubner.
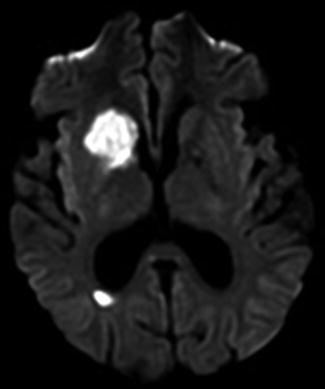

Fig. 5
Fifty-five-year-old male with history of atrial fibrillation presented with new onset seizures. Diffusion-weighted MRI obtained 3 h after onset of symptoms demonstrates restricted diffusion in the caudate nucleus, anterior limb of the internal capsule, and anterior aspect of the putamen and globus pallidus consistent with acute infarct in the expected vascular distribution of the recurrent artery of Heubner. There is an additional small acute infarct in the right periatrial white matter
Normal Variants of the Anterior Cerebral Arteries
The azygos anterior cerebral artery is a rare variant of the anterior cerebral artery involving a common trunk in the A2 segment (above the anterior communicating artery) with a prevalence of 0.2–4.0 % [14, 27]. Baptista distinguished three types of this anatomical variant: (1) “unpaired” ACA or true azygos artery (Type I anomaly) in which a single unpaired ACA provides branches to both cerebral hemispheres; (2) “bihemispheric” ACA (Type II anomaly) in which the A2 segment of one ACA sends branches across the midline, while the contralateral A2 segment either is hypoplastic or terminates early in its course toward the genu of the corpus callosum; and (3) “accessory” ACA (Type III anomaly) where there is an additional vessel arising from the anterior communicating artery accompanied by two hypoplastic A2 segments [27]. The most common anomaly noted by Baptista was the Type III anomaly, while the Type I anomaly was observed in only 1 of the 381 brain specimens examined by him [27]. An azygos anterior cerebral artery is associated with a large number of cerebral anomalies including agenesis of corpus callosum, porencephalic cysts, hydranencephaly, lobular holoprosencephaly, septo-optic dysplasia, and saccular aneurysms. Being aware of the presence of an azygos ACA is important, as an occlusion of this vessel can result in infarcts involving the bilateral medial cerebral hemispheres [14].
Trifurcation of the anterior cerebral artery, defined as the presence of three A2 segments of the ACA, is seen in 2–13 % cases and most likely represents persistence of the median callosal artery [14]. In the presence of thromboembolic disease, hypoplasia or absence of the A1 segment of the ACA typically results in a decreased collateral supply and consequently an increased risk of infarction [14].
Clinical manifestations of unilateral ACA territory infarction are spastic hemiparesis of the contralateral lower limb and to a lesser degree paresis of the arm, with the face and tongue largely spared [26, 28, 29]. Sensory dysfunction, particularly proprioceptive and discriminative impairment of the contralateral lower limb, may be present but is usually mild. Other manifestations of unilateral ACA territory infarction include speech disturbance with initial mutism, transcortical aphasia, apraxia, apathy, abulia, incontinence, and disinhibition [26]. Bilateral ACA territory infarction causes a neurologic syndrome characterized by profound akinetic mutism, paraparesis, and poor recovery [26].
Middle Cerebral Artery
The MCA has the largest vascular territory of all of the major intracranial arteries and is the vessel most commonly affected by cerebrovascular accidents [30]. Cortical branches of the MCA supply two-thirds of the lateral surface of the cerebral hemisphere with the exception of a thin 1-in. strip near the vertex that is supplied by the ACA and the occipital and posteroinferior parietal lobes that are supplied by the PCA (Fig. 1). Infarcts that occur in different segments of the MCA lead to diverse neurologic outcomes. The following subsections detail the specific MCA territories supplied by the various segments of the MCA and their correlation to specific neurologic deficits.
Horizontal (M1) Segment
The horizontal/M1 segment of the MCA arises from the carotid terminus and extends laterally where it bifurcates or trifurcates just before it enters the Sylvian fissure. The anterior temporal artery arises from the M1 segment and supplies the tip of the temporal lobe [4]. Lateral lenticulostriate arteries are deep penetrating branches of the M1 segment that supply the superior part of the head and body of the caudate nucleus, the putamen, and the external capsule [4, 31].
Complete proximal M1 occlusions (Fig. 6) result in sensory, motor, language, and executive function deficits due to disruption of both MCA cortical representations and basal ganglia circuit structures, while distal MCA occlusions spare the lateral lenticulostriate vascular territory but involve the distributions of both the superior and inferior division branches (see below).
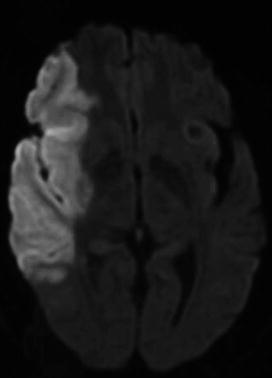

Fig. 6
Sixty-seven-year-old female with left hemiplegia last seen well 9 h prior to presentation in the emergency room. Diffusion-weighted MRI (DWI) demonstrated restricted diffusion within the right frontal and temporal lobe consistent with acute infarct in the vascular distribution of the superior and inferior divisions of the right middle cerebral artery
Insular (M2) Segments
In the majority of cases, the MCA divides into two trunks – superior and inferior – although occasionally it trifurcates into superior, middle, and inferior divisions [3, 4, 30, 31]. The superior division supplies the frontal and peri-Rolandic regions including the anterior parietal lobe [30]. The inferior division supplies the lateral temporal and inferior parietal lobes [30]. Figures 7 and 8 demonstrate acute infarcts in the vascular territories of the superior and inferior divisions of the MCA respectively.
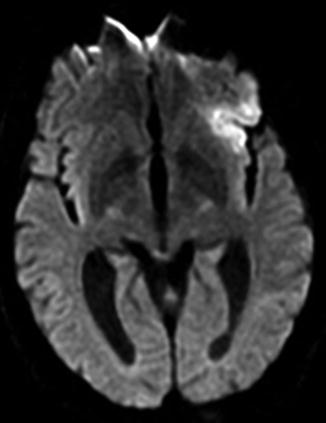
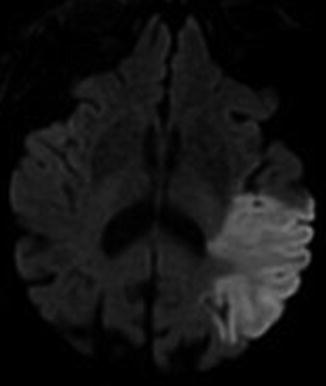

Fig. 7
Sixty-six-year-old male with right facial droop and expressive aphasia. Diffusion-weighted MRI (DWI) of the brain obtained 4 h after symptom onset shows an acute infarct involving the left frontal operculum and left anterior insula in the expected vascular distribution of the superior division of the left middle cerebral artery

Fig. 8
Eighty-nine-year-old female with new onset receptive aphasia. Diffusion-weighted MRI (DWI) of the brain obtained 12 h after symptom onset shows an acute infarct involving the left temporal lobe in the expected vascular distribution of the inferior division of the left middle cerebral artery
Opercular (M3) Segments
Cortical (M4) Segments
Normal Variants of the Middle Cerebral Arteries
An early bifurcation or trifurcation of the M1 segment close to its origin at the ICA is a common finding and is of no definite clinical significance [14]. A middle cerebral artery branch arising from the distal internal carotid artery is called a “duplicated” MCA, while a vessel arising from the ACA and coursing parallel to the M1 segment of the MCA is called an “accessory MCA” [14]. The frequency of accessory MCA is 0.3–0.4 %, while that of MCA duplication is 0.2–2.9 % [14]. The accessory MCA supplies the territory of the orbitofrontal, prefrontal, precentral, and/or central arteries [32]. The duplicated MCA supplies the territory of the lateral part of the orbital surface of the frontal lobe and the anterior and/or middle temporal territories. In the setting of MCA infarcts, the accessory MCA can provide collateral flow to the anterior frontal lobe, but it cannot supply enough flow to the main MCA territory [32]. Similarly, the duplicated MCA can provide collateral flow to the anterior temporal lobe, but it cannot supply enough flow to the main MCA territory [32].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree