Fig. 1
Longitudinal (a) and transverse (b) images of the left thyroid lobe on ultrasound. The maximum length (L), width (W), and depth (D) of each lobe are measured to calculate the thyroid volume
3.3 The Rationale for Dosimetry-Guided Therapy
Opponents to fixed activity algorithms point out that individualized dose calculation is important since there is no consistent correlation between TV and RAIU. Standard values for RAIU and T eff should not be assumed, bearing in mind that the computed T eff of 131I in the thyroid gland may actually range from 1.6 to 7.5 days as shown in a study by Berg et al. (1996). Treatment with fixed 131I activities may lead to unnecessary exposure for the patient or conversely, insufficient delivered dose to effect a cure. Outcomes cannot be predicted unless a sufficiently large dose is administered to all patients, some of whom will be over-treated. Rather, the optimal approach should be to eradicate hyperthyroidism with the lowest effective 131I activity.
3.4 Outcome Data
Published data on treatment with fixed activity regimens show higher rates of cure (defined as euthyroidism or hypothyroidism) with larger activities prescribed. Allahabadia et al. (2001) reported cure rates of 67 % in 443 hyperthyroid patients treated with 185 MBq 131I, and 85 % in 370 patients treated with 370 MBq. First-year hypothyroidism rates were 41 % and 61 % respectively. In another large study by Boelaert et al. (2009), 1278 hyperthyroid patients were treated with initial empirical 131I activities of 185, 370 and 600 MBq, with some patients requiring repeat treatment at 6 months. After a minimum follow-up of 1 year, cure rates were 63 %, 75 %, and 84 %, respectively. The corresponding first-year hypothyroidism rates were 38, 49, and 60 %.
For personalized dosimetry calculated on the basis of an intended absorbed dose to the thyroid, target doses of at least 200–250 Gy are recommended in order to achieve consistent results. Howarth et al. (2001) treated 58 GD patients with an intended absorbed dose of either 60 or 90 Gy. At 6 months follow-up, remission was achieved in only 39 % of the former group of patients and in 41 % of the latter group. At the other extreme, Willemsen et al. (1993) who treated 43 patients with recurrent GD aiming for a tissue-absorbed dose of 300 Gy, reported a 100 % cure rate at 1 year. 93 % of patients had become hypothyroid by 18 months post-therapy. In the study by Reinhardt et al. (2002), 224 GD patients were treated with target doses of 150, 200, or 300 Gy. At 15 month follow-up, remission rates were 73 %, 77 %, and 92 %, respectively; the corresponding hypothyroidism rates were 23, 26, and 42 %.
For individualized calculations based on the “activity per gram” method, Iagaru and McDougall (2007) recommend prescriptions in the range of 150–200 μCi 131I per gram thyroid mass, which is expected to result in <10 % of patients requiring repeat treatment.
4 Adjunctive Treatment with Anti-Thyroid Drugs
Anti-thyroid drugs inhibit iodine organification and iodotyrosyl coupling, and are frequently prescribed before 131I therapy to deplete pre-formed stores of thyroid hormone, thus reducing the risk of transient exacerbation of thyrotoxic signs and symptoms. However, the drawback of adjunctive treatment with thyrostatic drugs is a reduction in the efficacy of 131I in eliminating the hyperthyroid state.
4.1 Concurrent Treatment with ATD
Studies of 131I kinetics in the thyroid gland, based on laborious uptake measurements have shown that concurrent treatment with thiamazole (TMZ) results in an average reduction of 35 % in RAIU, and a 2.5 times reduction in T eff which is restored within 1–2 days after discontinuation of TMZ (Dunkelmann et al. 2007).
Results of several studies leave little doubt that thyrostatic medication when prescribed concurrently with 131I increases the risk of RAI failure. Sabri et al. (1999) found that hyperthyroidism was successfully eliminated 12 months after RAI in only 49 % of patients who were maintained on carbimazole (CBZ) while undergoing 131I therapy, whereas in the group of patients who were taken off CBZ for a median period of 17 days before RAI, 93 % were cured. This marked difference in cure rate was observed in spite of the fact that a higher administered dose had been prescribed for the former group of patients in order to compensate for the reduced uptake and T eff of 131I in the thyroid gland. Bonnema et al. (2006) treated 75 hyperthyroid patients with methimazole (MMI) until they were euthyroid before 131I therapy. Patients were then randomly assigned to continue taking MMI, or to stop MMI 8 days before RAI. The administered 131I activity was calculated taking into account the TV and T eff of 131I in the thyroid gland. At 1 year, cure was achieved in 44 % of patients who continued taking MMI, and in 61 % of the patients who had discontinued MMI. Walter et al. (2006) reported a substantial reduction in cure rate at 1 year in patients who were on CBZ during the time of 131I treatment (42.6 %), compared to patients who had never been on CBZ (81.4 %) and those who had been taken off CBZ 3 days before (83.3 %). Similarly, Dunkelmann et al. (2007) treated 316 GD patients with individualized doses of 131I ranging from 125 to 250 Gy. The cure rates among patients who were maintained on TMZ during 131I treatment, patients who discontinued TMZ 3 days before, and those who had never received TMZ were 55 %, 87 %, and 82 %, respectively.
4.2 Withdrawal of ATD Before RAI
Since thyrostatic drugs taken concurrently with 131I negatively impacts cure rates, what is the optimal period of withdrawal before RAI? Recently published guidelines from the American Thyroid Association (ATA) (Bahn et al. 2011) recommend withdrawal of MMI for 3–5 days before 131I administration. In a prospective trial, 61 patients with GD were randomized to treatment with RAI alone, or to pre-treatment with MMI which was discontinued 4 days before RAI. Patients were prescribed 200 μCi 131I per gram thyroid mass, corrected for 24-h RAIU. At 1 year, there was no difference between the two groups of patients in terms of persistent hyperthyroidism (15.6 % versus 13.8 %), euthyroidism (28.1 % versus 31.0 %), or hypothyroidism (56.3 % versus 55.2 %) (Andrade et al. 2001). In another prospective randomized study, similar cure rates were observed among GD patients who discontinued MMI 6 days prior to RAI, and those who received RAI alone without pre-treatment. The time taken to achieve hypothyroidism was also not significantly different between the two groups (Braga et al. 2002).
As for propylthiouracil (PTU), early studies have shown that the drug, even though withdrawn for at least 4 days before RAI, is associated with a significant increase in RAI treatment failure (Hancock et al. 1997; Tuttle et al. 1995). It is widely believed that the effect of PTU on 131I kinetics may be more protracted compared with the imidazole derivatives. Data from two non-randomized studies comparing the effect of PTU versus MMI on the therapeutic efficacy of RAI showed significantly lower cure rates in patients who had been pre-treated with PTU (Imseis et al. 1998; Santos et al. 2004). Imseis et al. (1998) reported that patients pre-treated with MMI had similar cure rates (61 %) compared to non-pre-treated patients (66 %), whereas PTU pre-treatment was associated with a significantly lower cure rate (24 %). The two drugs had been discontinued for a similar period of time prior to RAI therapy. The Royal College of Physicians (2007) Workgroup has recommended discontinuing PTU for at least 2 weeks before RAI, otherwise a larger 131I dose may be needed to overcome the protective effect.
Contrary to these findings, a recent retrospective review by Kobe et al. (2008) showed that 131I was equally effective in patients pre-treated with PTU (1 year cure rate 100 %), CBZ, or MMI (cure rate 96 %), all withdrawn 2 days before RAI. In another study, cure rates were also not significantly different among hyperthyroid patients pre-treated with CBZ or PTU, both discontinued 1 week before RAI treatment (Boelart et al. 2009).
4.3 Resumption of ATD After RAI
The issue of whether or not to resume ATD after RAI has been less extensively investigated. In a randomized trial of 149 hyperthyroid patients rendered euthyroid with MMI which was stopped 4 days before RAI, re-starting MMI 7 days later was not associated with poorer outcome compared to the group of patients in whom MMI was not reinstated (Bonnema et al. 2003). Bahn et al. (2011) propose re-starting MMI 3–7 days after RAI, and tapering the dosage over 4–6 weeks as thyroid function returns to normal.
4.4 Is Adjunctive ATD Treatment Clinically Relevant?
Given that clinically evident worsening of hyperthyroidism after RAI occurs on average in <1 % of patients (McDermott et al. 1983), the relevance of pre-treatment with ATD has been questioned. Exacerbations after RAI therapy have been reported in patients with or without drug pre-treatment. In a randomized trial involving 42 patients, Burch et al. (2001) found that in pre-treated patients, a transient rise in serum thyroid hormone levels occurred after RAI which they attributed mainly to a ‘rebound’ following withdrawal of the ATD, rather than to the administration of 131I. Conversely, further elevation of thyroid hormone levels did not occur in the majority of patients who had not received ATD. Instead, the levels declined rapidly within the first 2 weeks after RAI. At first glance, this would seem to suggest that pre-treating patients with thyrostatic medication might actually be more harmful than not prescribing them at all. However, it should be noted that the transient surge in thyroid hormone levels in the pre-treated patients arises from a lower baseline and is generally not manifested clinically.
Routine use of adjunctive ATD with RAI is probably unnecessary in the majority of patients, but should be considered when treating high-risk groups which include the elderly, patients who are extremely symptomatic, those with fT4 levels 2–3 times above the upper limit, and those with cardiovascular co-morbidities (Burch et al. 2001; Mijnhout and Franken 2008; Bahn et al. 2011).
5 Patient Characteristics and Predictors of Cure
The pre-treatment identification of certain clinical profiles that are expected to have an adverse effect on cure rates would allow clinicians to adjust their 131I dose prescriptions toward the higher end of the scale. Several clinical parameters that may potentially influence the outcome of RAI therapy have been investigated. Unfortunately, the findings among studies are occasionally conflicting. In two studies, male patients were found to have lower cure rates compared to females (Allahabadia et al. 2001; Boelart et al. 2009). However, other studies report no adverse influence of patients’ age and gender on outcome (Bonnema et al. 2006; Zantut-Wittmann et al. 2005; Andrade et al. 2001; Kobe et al. 2008). An inverse correlation between TV and treatment outcome has been documented (Allahabadia et al. 2001; Zantut-Wittmann et al. 2005; Boelart et al. 2009), whereas this association was not observed by others (Bonnema et al. 2006; Sabri et al. 1999). Higher pre-treatment levels of serum fT4 or fT3 were found to be associated with lower cure rates by some investigators (Allahabadia et al. 2001; Alexander and Larson 2002; Andrade et al. 2001; Boelart et al. 2009) but not by others (Zantut-Wittmann et al. 2005; Sabri et al. 1999). Baseline TSH-receptor antibodies (TRAb) levels were not correlated with outcome in two studies (Andrade et al. 2001; Sabri et al. 1999). However, Chiovato et al. (1998) reported that patients with high pre-treatment thyroid-stimulating antibody (TSAb) levels were more likely to fail to respond to therapy.
Contrary to the presumption that a high pre-treatment RAIU value bodes well for treatment success, several studies have demonstrated a poorer outcome for patients presenting with high uptake values (Walter et al. 2004; Kristoffersen et al. 2006; Bonnema et al. 2006). Possible explanations that have been postulated for this apparently paradoxical relationship include stunning of the thyroid gland by the pre-treatment uptake test dose, and an inverse correlation between RAIU and the inherent radiosensitivity of the gland (Walter et al. 2004). A similar inverse correlation has been reported for pre-treatment uptake of 99mTc pertechnetate and RAI outcome (Zantut-Wittmann et al. 2005).
6 Adjuvant Treatment with Lithium
Adjuvant treatment with lithium was investigated by Bal et al. (2002) in a randomized controlled trial. 316 patients with hyperthyroidism were randomized to treatment with RAI alone, or RAI with lithium given for 3 weeks beginning on the day of receiving RAI. The use of lithium was neither associated with an improved cure rate, nor with a reduction in number of doses of RAI required to achieve cure. Moreever, treatment with lithium was not without side effects: about 10 % of patients experienced nausea, vomiting, giddiness, and mild diarrhea.
On the other hand, Bogazzi et al. (2010) have recently reported that a short course of lithium may be beneficial in GD patients treated with RAI. This was a large retrospective cohort study involving 298 patients who were given lithium (900 mg daily) for 12 days around the time of RAI treatment. Compared to 353 patients who did not receive adjuvant lithium, those treated with lithium had a higher cure rate (91 % versus 85 %) and a shorter median time to cure (60 days versus 90 days). The addition of lithium also blunted the transient rise in serum fT4 levels associated with ATD withdrawal or RAI. The incidence of side effects did not differ in the two groups.
In the absence of further data supporting an adjuvant role of lithium, the therapy committee of the European Association of Nuclear Medicine does not recommend routine addition of lithium to a RAI regime, though its use may be considered if 24-h thyroid uptake is <20 % (Stokkel et al. 2010).
7 RAI and Graves’ Ophthalmopathy
There is increasing evidence that ophthalmopathy in Graves’ disease results from autoimmunity targeting TSH-R expressed on orbital fibroblasts. Activated orbital fibroblasts secrete a variety of cytokines which provoke T cell recruitment into the orbit, and complex intercellular interactions ultimately result in fibroblast proliferation, increased adipogenesis and accumulation of hydrated glycosaminoglycans within the orbit (Lehmann et al. 2008). A schematic illustration of the pathogenic process is shown in Fig. 2.
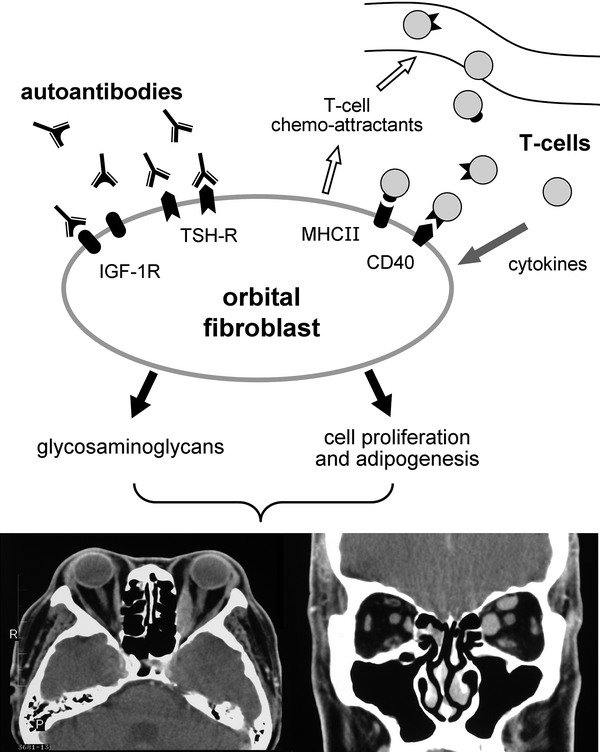

Fig. 2
Key steps in the pathogenesis of GO. Autoantibodies (GD-IgG) induce orbital fibroblasts to release chemoattractants, triggering the migration of T-cells into the orbit. The interaction of T-cells and orbital fibroblasts fosters reciprocal activation, thus enhancing the production of cytokines, fibroblast proliferation, and synthesis of glycosaminoglycans. Increased adipogenesis and accumulation of glycosaminoglycans within the orbit is responsible for the clinical manifestations of GO. Transverse and coronal CT images in a patient with bilateral proptosis and enlargement of the medial rectus muscles and the left superior rectus
RAI treatment has been associated with worsening of eye disease in patients with pre-existing GO, as well as de novo development of GO in patients without ocular manifestations before treatment. Aggravation of eye disease is most likely due to a post-treatment surge in serum TRAb levels which has been shown by Laurberg et al. (2008) to occur exclusively after treatment with RAI.
Three large randomized trials that specifically address the orbital changes of GD as the primary outcome after treatment have shown that RAI treatment carries a small but definite increased risk of developing or progression of GO compared to treatment with ATD or thyroidectomy. The study by Tallstedt et al. (1992) revealed that among 114 patients treated for GD, the frequency of development or progression of GO was similar in patients treated with ATD (10 %) or sub-total thyroidectomy (16 %), but was significantly higher in those treated with 131I (33 %). The risk of progression of GO was found to be higher with increasing pre-treatment T3 levels.
In the study by Bartalena et al. (1998), 450 patients with mild GO or no GO were randomly assigned to treatment with RAI alone, MMI, or RAI followed by a 3 month course of oral prednisolone. (Mild GO was defined as proptosis of <22 mm, intermittent or no diplopia, absence of optic neuropathy and mild conjunctival and periorbital inflammation). Among the 150 patients treated with RAI alone, progression of GO occurred in 23 (15 %). This deterioration was mostly transient and mild, but 8 patients subsequently required specific treatment for GO. Among 148 patients treated with MMI, progression was observed in only four (3 %). None of the 145 patients treated with RAI plus prednisolone experienced worsening of GO; conversely, the eye signs regressed in 50 out of the 75 (67 %) patients in this group who had GO at baseline. Another pertinent finding derived from this study was a significantly higher prevalence of smokers in the group of patients who experienced ocular progression compared to the group without progression (83 % versus 50 %).
The most recent study by Traisk et al. (2009) involved 316 patients with newly diagnosed GD, randomized to treatment with RAI or MMI. One year after treatment, development, or worsening of GO occurred in 31 % of patients given RAI, and in 16 % of those treated with MMI. Similar to the report by Bartalena et al. (1998), these investigators also established smoking as a risk factor for GO, independent of treatment modality.
Apart from high baseline levels of T3 and cigarette smoking, post-treatment hypothyroidism has also been identified as a risk factor for ocular progression (Acharya et al. 2008), underscoring the importance of early follow-up of patients post-RAI so that hypothyroidism can be detected and promptly corrected. Patients with high TRAb levels and uncontrolled hyperthyroidism are also thought to be at increased risk of ocular progression (Tanda et al. 2008).
It is important to note that the majority of studies involved only patients presenting with mild or minimally active GO. The lack of studies involving patients with moderate or severe GO and CAS >3 suggests that these patients are treated either with thyroidectomy or are placed on long-term ATD and referred for RAI only when the eye disease has ‘burnt out’.
With Bartalena et al. (1998) having convincingly proven the efficacy of prednisolone prophylaxis in preventing deterioration of mild GO after RAI, the ATA has recommended that patients with GO which is mild and active and who smoke should receive glucocorticoid prophylaxis if RAI is the chosen treatment modality. For non-smoking patients with mild and active GO, decisions regarding concurrent steroid use should be made taking into consideration the patients’ overall health risk, weighing the risk of post-RAI ocular deterioration versus the risk of developing complications from steroid use. Glucocorticoid prophylaxis is not required in patients with inactive GO, regardless of smoking history (Bahn et al. 2011).
8 Treatment of Childhood Graves’ Disease
Relative paucity of data describing the optimal dose regimen and the outcome of RAI treatment in children with GD, together with the concern of a potential increase in thyroid malignancies associated with 131I, largely account for clinicians’ hesitancy in considering definitive treatment with RAI, and ATD is the initial treatment of choice in most centers. The optimal duration of ATD use prior to definitive treatment has not been determined and many children are maintained on prolonged courses of ATD in the hope of achieving remission. Unfortunately, long-term remission with ATD is difficult to attain in childhood GD. In one study, out of 51 children who were treated with ATD for 2 years, only 15 (29 %) were in remission 12 months after discontinuation of medication (Glaser and Styne 2008). In another large prospective study of 147 children with GD treated with carbimazole for a median period of 25 months, 59 % and 68 % of children relapsed at 1 year and 2 years respectively after discontinuation of carbimazole (Kaguelidou et al. 2008).
On this note, several investigators have recently sounded an alert regarding the safety profile of propylthiouracil, a hitherto commonly prescribed anti-thyroid medication. Recent reviews on the hepatotoxic effects of ATD reveal that PTU-associated hepatotoxicity is encountered more often than initially thought, particularly among children and adolescents, in some cases resulting in liver failure (Rivkees and Szarfman 2010). Thus, the practice of prescribing PTU for childhood GD should be discontinued in favor of MMI or CBZ (Rivkees and Mattison 2009; Malozowski and Chiesa 2010; Bauer 2011).
In contrast to adult GD, there are no studies comparing treatment outcomes using fixed activities of 131I versus calculated doses in children. Rivkees and Cornelius (2003) reviewed the treatment outcome in 31 children and adolescents with GD who had received RAI between the ages of 7–18 years. After a minimum follow-up period of 30 months, hyperthyroidism persisted in two out of seven (28.6 %) patients who had received 80–120 μCi of 131I per gram thyroid mass, and in three out of 8 (37.5 %) patients who had received 200–250 μCi g−1, whereas all 16 patients who were prescribed 300–405 μCi g−1 had become euthyroid or hypothyroid. In a more recent retrospective review of 22 patients treated with 100 μCi of 131I per gram thyroid mass between the ages of 5–19 years (mean activity administered was 11 mCi), 73 % became hypothyroid after a single RAI dose (Pinto et al. 2007). In another retrospective study, a fixed activity of 15 mCi was found to be effective in ablating the thyroids of children and adolescents aged between 8–19 years. Hypothyroidism developed in 38 out of 40 patients after a single 131I dose (Nabesio et al. 2002).
The issues of radiation-induced carcinogenesis and genetic damage were addressed by Read et al. (2004) who collected the outcome data of 116 patients who had received RAI for GD between the ages of 3–19 years. The average length of follow-up was 26–36 years. None of these patients developed thyroid cancer, and no adverse effects on subsequent pregnancies and offspring were observed. It should be noted that the majority of their patients were treated with low 131I activities averaging 3–4 mCi. Long-term safety data involving a larger number of patients treated with larger 131I doses remains to be established.
Anecdotal reports of thyroid cancer induction in 4 children who had previously been treated with low doses of 131I (a 5-year-old child treated with 50 μCi g−1, a 9-year-old treated with 5.4 mCi, an 11-year-old treated with 1.25 mCi, and a 16-year-old treated with 3.2 mCi) have led to the proposal to prescribe ablative 131I doses rather than low doses for childhood GD (Rivkees and Dinauer 2007; Krassas 2004). Recent guidelines from the ATA propose a minimum 131I therapy dose of 150 μCi per gram of thyroid tissue in order to achieve hypothyroidism rates of 95 %. For goiters larger than 80 g, 131I may not provide effective ablation, and thyroidectomy is preferred (Bahn et al. 2011).
Whereas there is no absolute cutoff for age below which 131I treatment is contraindicated, considering that the thyroid glands of children below the age of 5 years are at increased susceptibility to the carcinogenic effect of radiation, it has been suggested that it may be safer to avoid using RAI in this age group (Rivkees and Dinauer 2007; Stokkel et al. 2010; Bahn et al. 2011). For children aged between 5 and 10 years, Bahn et al. (2011) recommend limiting the administered 131I activity to <10 mCi.
9 Complications and Side Effects of 131I Therapy
Weight gain in excess of pre-morbid weight occurs upon elimination of hyperthyroidism, when metabolic rate normalizes without concomitant reduction in appetite. Thus, patients should be given advice regarding moderation of food intake during follow-up. In a group of hyperthyroid patients, Dale et al. (2001) reported a mean post-treatment gain of 3.6 kg per year over 4 years, whereas Brunova et al. (2003) showed that most of the increase occurs during the initial 2 years after RAI. Patients who become hypothyroid requiring T4 replacement tend to gain more weight than those who remain euthyroid after treatment. Among those with post-treatment hypothyroidism, RAI was not associated with greater weight gain compared to treatment with sub-total thyroidectomy (Tigas et al. 2000).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







