Fig. 1
Immunoscintigraphy (anterior and posterior views) performed in a MTC patient. The images show good tumor targeting in liver and brain metastases
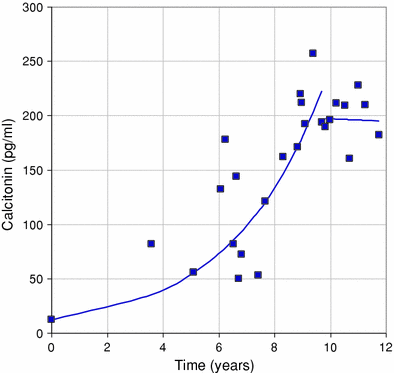
Fig. 2
Post-RIT biological response. The figure shows stabilization, extending by 2 years, of calcitonin serum level after RIT. The Ct DT was 2.3 years before RIT
5.2 Targeted Therapy with Multikinase Inhibitors
It has been clearly established that the RET protein plays an important role in MTC (Romei et al. 1996), and thus could be an appropriate target for molecular therapy. Other signaling components can be involved in MTC, such as epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF). Consequently, therapy with multikinase inhibitors has been evaluated in preclinical and clinical studies.
Three clinical studies have been conducted in a total of 30 patients with imatinib mesylate, which inhibits RET (Gross et al. 2006; de Groot et al. 2007; Frank-Raue et al. 2007). Severe toxicity was reported in one study and included nausea and vomiting, fatigue, rash, and laryngeal mucosal swelling (de Groot et al. 2007). Several other protein-kinase inhibitors have been evaluated. Axitinib, an inhibitor of VEGF receptor, was evaluated in 12 patients with some stabilization of disease results (Cohen et al. 2007). Sorafenib, a selective inhibitor of RET tyrosine kinases, was evaluated in 5 patients with a surprising complete response in one patient and partial in another, but serious toxicity reduced the dose by 50 % (Kober et al. 2007). Motesanib, an inhibitor of the VEGF, PDGF, and stem-cell factor (Kit) receptor, was evaluated in a phase II clinical study enrolling 91 patients with locally advanced or metastatic disease. The treatment-related adverse events included diarrhea, fatigue, hypothyroidism, hypertension, and anorexia. Interestingly, a stabilization of disease was observed in 81 % of evaluable patients and was durable (≥24 weeks) in 48 % (Schlumberger et al. 2009).
Finally vandetanib, which targets RET, VEGF, and EGF receptor tyrosine kinases, was first evaluated in 30 patients and showed a 20 % partial response rate, 30 % stabilization of disease, and 63 % having a biological response. Grade-3 adverse events were observed in 3 patients (Wells et al. 2007). Subsequently a randomized phase III trial was performed in 331 patients with advanced and metastatic medullary thyroid cancer. A progression-free survival gain of 11 months was observed in the treated group (predicted median of 30.5 months) as compared to the control group (median of 19.3 months) (Wells et al. 2012). Based on these results vandetanib was approved in 2012.
5.3 Radiopeptide Therapy
90Yttrium (90Y)-labeled, 1,4,7,10-tetra-azacyclododecane N,N0,N00,N000-tetraacetic acid (DOTA)-modified somatostatin analog, Tyr3-octreotide (TOC,) or [90Y-DOTA]-TOC, a somatostatin analog was evaluated in 31 patients with metastatic disease (Iten et al. 2007). Long-term renal toxicity was the main adverse event, with transient grade-1 observed in 2 patients and long-lasting grades-1 and -4 observed in 3 and 1 patients, respectively. The grade-4 renal toxicity occurred 25.8 months after treatment.
A post-therapeutic prolongation of calcitonin doubling time of at least 100 %, which is considered as a biological response criterion (Barbet et al. 2005), was found in 58 % of patients. The median survival was 15.7 months (range 1.4–107.0 months) from the time of first [90Y-DOTA]-TOC treatment. Responders had a significantly longer median survival as compared with nonresponders, from time of [90Y-DOTA]-TOC treatment (74.5 months, range 15.7–107.0 vs. 10.8 months, range 1.4–85.0, P = 0.02).
It is difficult to draw any valid conclusions about survival benefit observed with multikinase inhibitors and radiopeptide therapy in the absence of data on real tumor growth rate before treatment. Indeed, tumor growth rate is highly variable in patients with documented metastases of MTC, with a very slow progression for some patients that can simulate a stabilization extending over some years. The tumor growth rate before therapy can be evaluated by calcitonin doubling time, which appears to be the best prognostic indicator to select patients before any investigational treatment (Barbet et al. 2005). This would permit patients to be selected on the basis of a calcitonin doubling time <2 years, which means a rapidly progressing disease with a short life expectancy.
6 Conclusion
For patients with metastatic MTC chemotherapy has been associated with severe toxicity, with no convincing survival benefit. Some innovative treatment modalities have been recently evaluated, including targeted therapy with multikinase inhibitors, radiopeptide therapy, and pretargeted RIT. This last modality showed some convincing clinical efficacy, with a significant survival benefit in patients with rapidly progressing metastatic disease, compared to untreated patients. Vandetanib demonstrated therapeutic clinical efficacy in a phase III trial. However, due to some potential severe toxicity, its use should be restricted to patients with rapidly progressive disease, as documented by a calcitonin doubling time of less than 2 years.
References
Bardies M, Bardet S, Faivre-Chauvet A et al (1996) Bispecific antibody and iodine-131- labeled bivalent hapten dosimetry in patients with medullary thyroid or small-cell lung cancer. J Nucl Med 37:1853–1859PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







