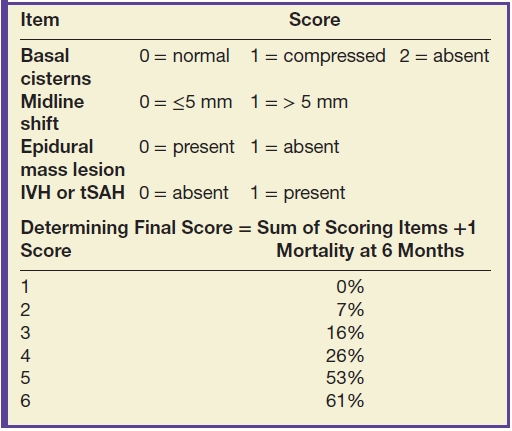
Grading of severity of CT findings has traditionally relied on the Trauma Coma Data Bank (TCDB) classification, also known as the Marshall classification (11) (Table 3.2). However, this classification system, which was initially developed from data on severely injured patients, has been shown to have poor generalizability. In the recent decade, an updated classification has been devised, the Rotterdam CT score (Table 3.3), although this is still primarily used in moderate-to-severely injured patients, to predict mortality rather than morbidity or disability (12).
Table 3.2 TRAUMA COMA DATA BANK (TCDB) OR MARSHALL CLASSIFICATION—OF CT ABNORMALITIES IN TBI
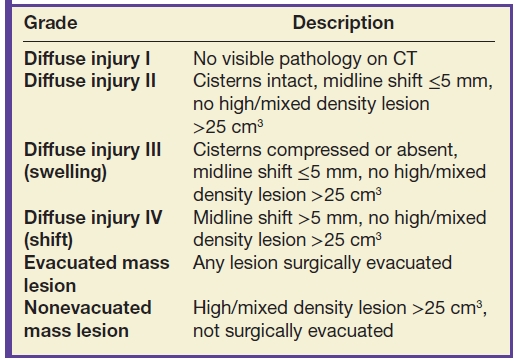
Table 3.3 ROTTERDAM CT SCORE—FOR PREDICTING MORTALITY AT 6 MONTHS POSTINJURY
Types of Injuries
TBI can be divided into primary and secondary injuries, both of which can include many different kinds of lesions. Primary injuries can be classified by mechanism or by location. Mechanisms can also be divided into focal (contact) versus diffuse (inertial) types of injury. Or, they can be divided into closed (blunt) versus open (penetrating) types of injury. However, for radiologic evaluation of TBI imaging findings, it is helpful to consider primary injuries by location, beginning at the outside of the head and progressing inward (Table 3.4).
Table 3.4 ALGORITHM FOR EVALUATING HEAD CT FOR TRAUMA—“OUTSIDE-TO-INSIDE” APPROACH
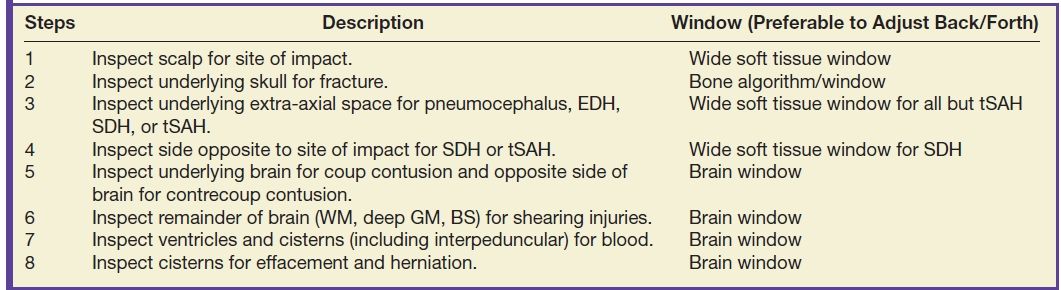
Scalp
Scalp injuries vary by mechanism and force and include laceration, contusion, and hematoma. The location of scalp injury is a helpful indicator to the side of impact and therefore location of “coup” injury. Scalp laceration may be obvious, with a soft tissue cleft that can extend to the bone. However, the presence of subcutaneous emphysema, even without a visible defect, can suggest laceration. Scalp swelling can consist of edema, contusion, and/or frank hematoma. Scalp hematomas after head injury are usually located under the galea aponeurosis (subgaleal or subaponeurotic). These collections can be diffuse and are not bounded by anatomic compartments. A subtype of scalp hematoma is the cephalohematoma, which can occur in newborns who have undergone delivery with the aid of forceps or other instruments. The cephalohematoma is subperiosteal in location, beneath the outer periosteal layer of the skull, and is therefore bounded by cranial sutures and is more localized. They often go undetected but may present later after the hematoma begins to resorb/calcify and the periosteum thickens, at which point the parents may notice a “bump” on the skull.
Skull
If scalp injury is identified, carefully inspect the adjacent skull to detect any underlying fracture. There are various types of fractures (Table 3.5), with different management implications. Probably of greatest concern are open or compound fractures that communicate with air. Fractures involving paranasal sinuses (with walls that abut the cranial vault) or temporal bone air cells are potential sites of intracranial communication (either as a route of infection or CSF leak). Skull base fractures are often open/compound fractures. In children, remember to inspect the sutures for possible diastatic sutural fractures. Depressed fractures may require surgical elevation if the bone is more than 5 mm depressed or impinging on important underlying structures such as the eloquent cortex or dural venous sinus. Beware of misinterpreting vascular grooves and vascular channels, which usually have a branching pattern, tend to be slightly “wavy” rather than sharp and straight, and often taper. They may also be symmetric. On cross section, vascular channels have a characteristic smooth, round lumen, usually with slightly sclerotic margins, and at some point are completely encircled by bone. Vascular grooves create indentations along the inner table of the skull, with sparing of the outer table.
Table 3.5 FRACTURE TERMINOLOGY
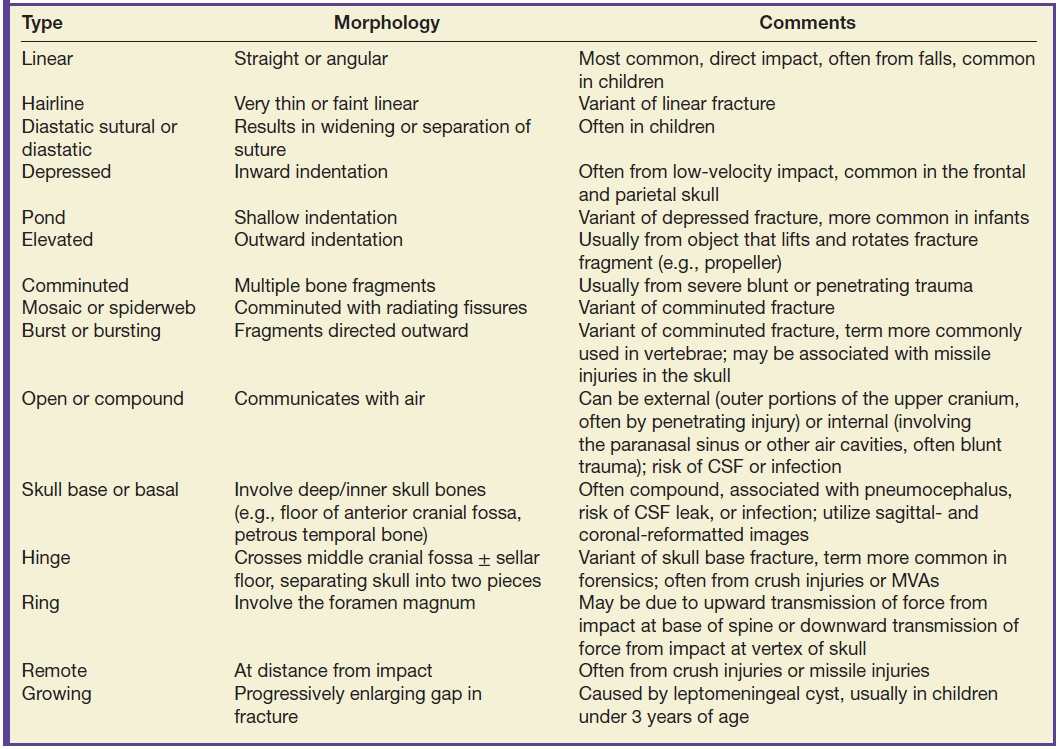
Traumatic extra-axial collections: epidural, subdural, subarachnoid, and intraventricular hemorrhages and subdural hygroma
There are several different types of extra-axial collections associated with TBI. In general, traumatic subarachnoid hemorrhage (tSAH) is more common than subdural hematoma (SDH), which is more common than epidural hematoma (EDH). They can also occur simultaneously (Fig. 3.1). It can sometimes be difficult to distinguish an EDH from SDH (in which case the term “extra-axial hemorrhagic collection” can be used), although there are typical features of each type of collection (Table 3.6). Both EDH and SDH can result in a lucid interval when the patient seems neurologically intact, before decompensating. It can be more difficult to discriminate between EDH and SDH in the subacute to chronic period, as the morphology of one may mimic the other. Small collections (particularly SDH) are often missed, due to their close proximity to the bone. Be aware that extra-axial hematomas can have diffusion restriction, which should not be mistakenly interpreted as epidural abscess or subdural empyema. Acute, subacute, or chronic dural enhancement can also occur after head trauma.
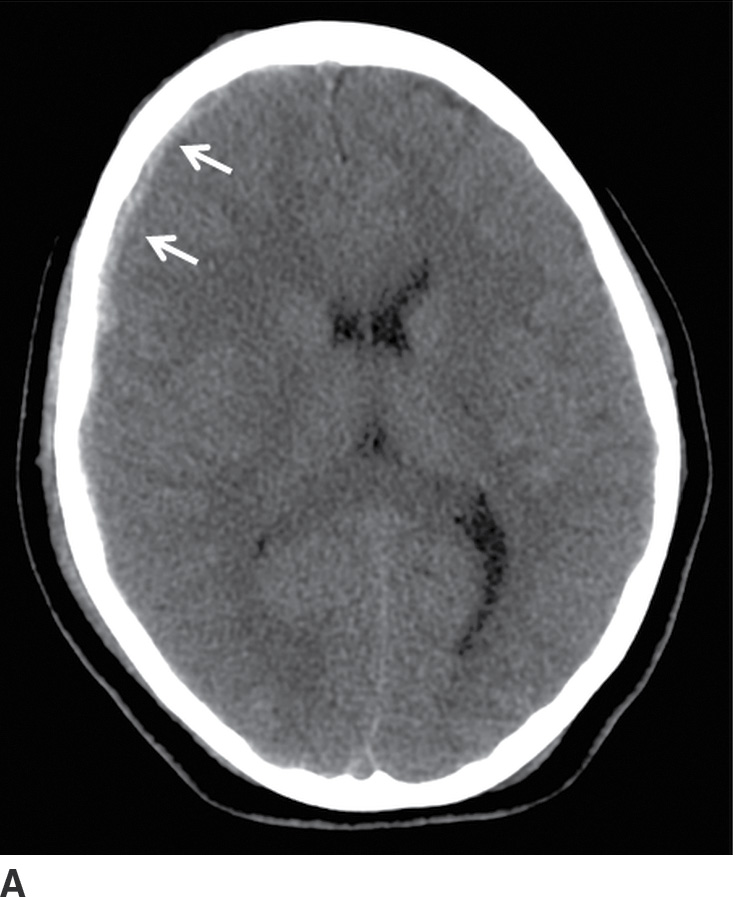
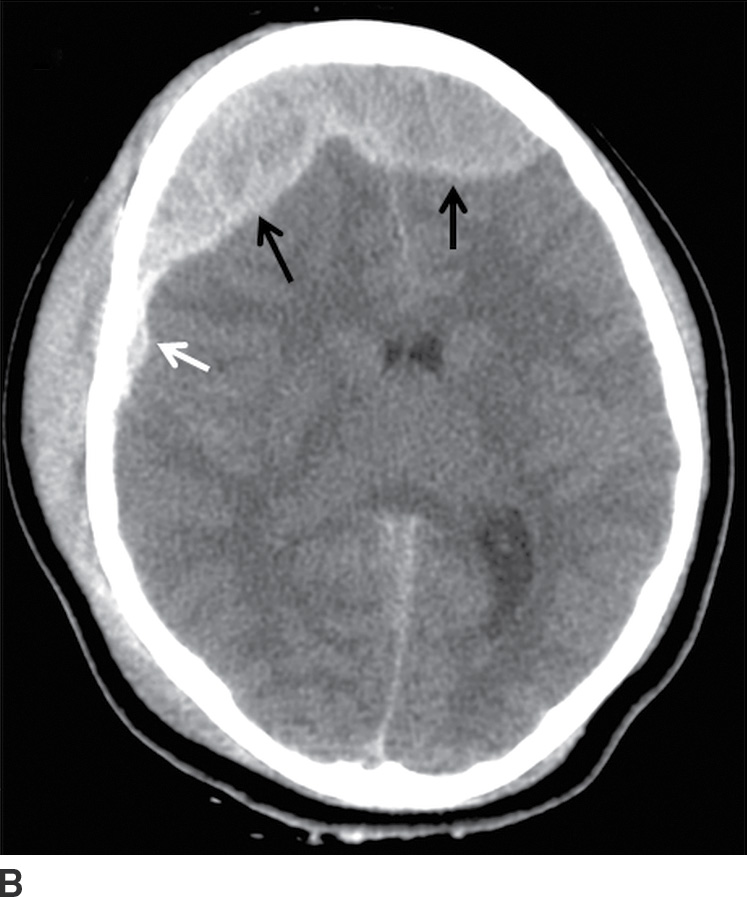
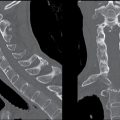
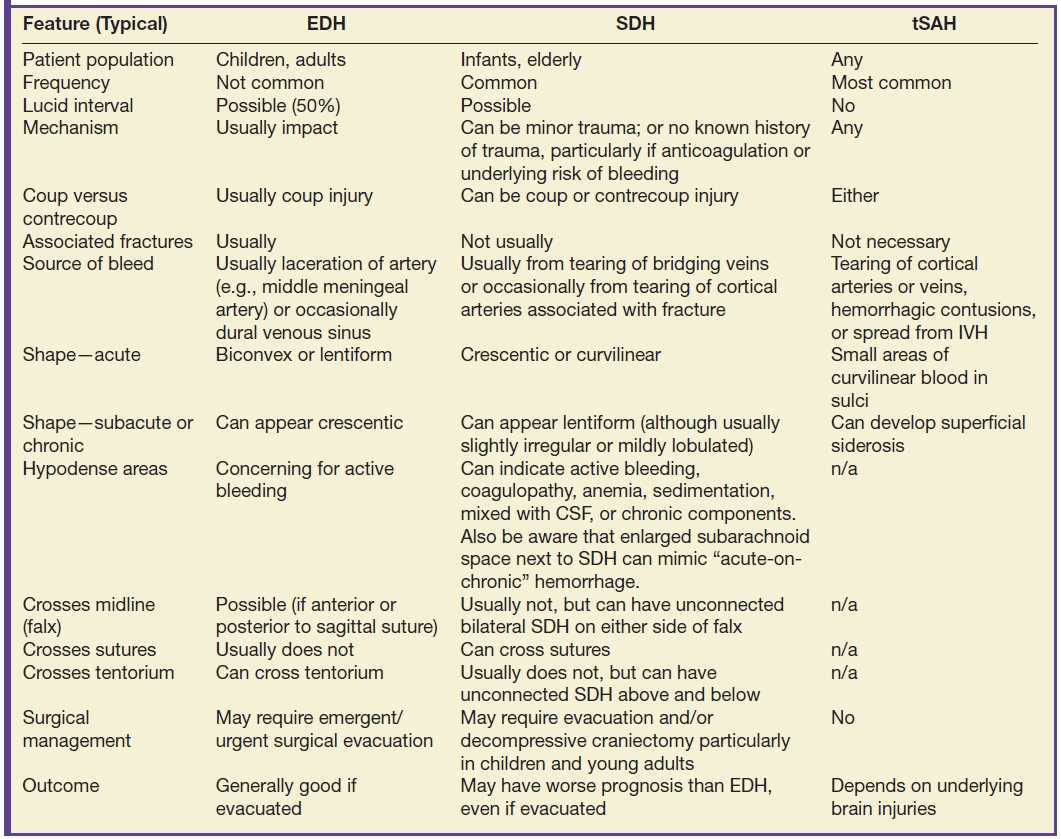
FIG. 3.1 SDH and EDH.Axial CT images 4 hours apart in a child involved in an MVA. A: Initial CT shortly after admission shows a small curvilinear/crescentic SDH over the right frontal and parietal convexity (arrows). Several hours later, markedly elevated ICP measurements were obtained from a ventricular catheter placed after the first scan. B: Second CT 4 hours later showed interval development of a large bifrontal epidural hematoma (confirmed at subsequent surgical decompression), in addition to prior SDH (arrow).
Table 3.6 DISTINGUISHING EDH, SDH, AND TSAH
Epidural hematomas
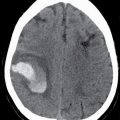
An EDH arises between the outer layer of the dura and the skull. The typical acute EDH usually occurs in children or adults, is associated with a fracture, and usually occurs from laceration of an artery (often the middle meningeal artery) or occasionally from laceration of a dural venous sinus. They are biconvex or lentiform in shape and are bounded by the cranial sutures, but not the dural reflections (Fig. 3.2). Therefore, they can cross the midline if they are anterior or posterior to the sagittal suture (Fig. 3.3) and can cross above or below the tentorium. The majority of EDHs arise from an arterial source and will develop more quickly than from a dural venous source. EDH from laceration of a dural venous sinus can extend on either side of the dural reflection and can bleed into adjacent compartments. The presence of hypodense areas, particularly if they have a “swirling” appearance, may indicate active bleeding and should prompt immediate communication to the clinical team (13). If evacuated promptly, the patient prognosis is often very good. Rapid enlargement of an acute EDH is unpredictable, and repeat CT may be warranted to ensure stability, even without clinical deterioration.
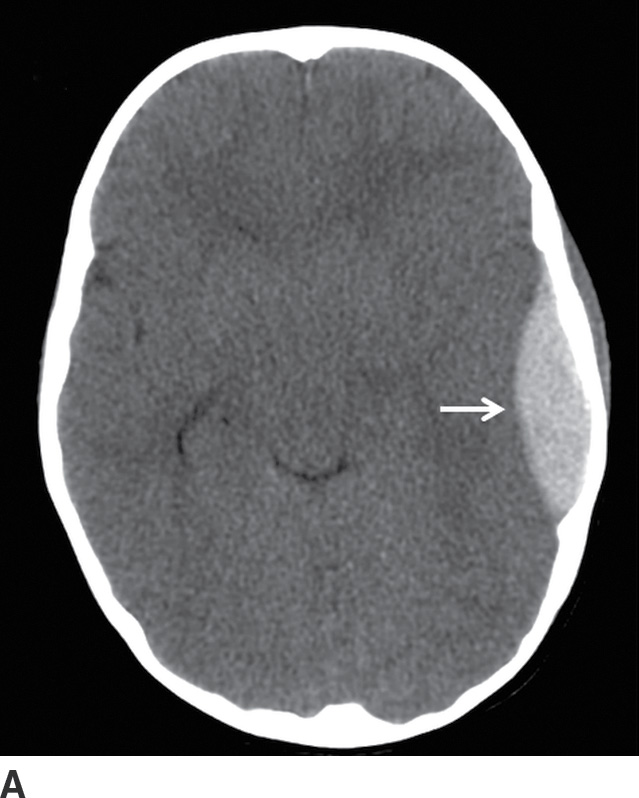
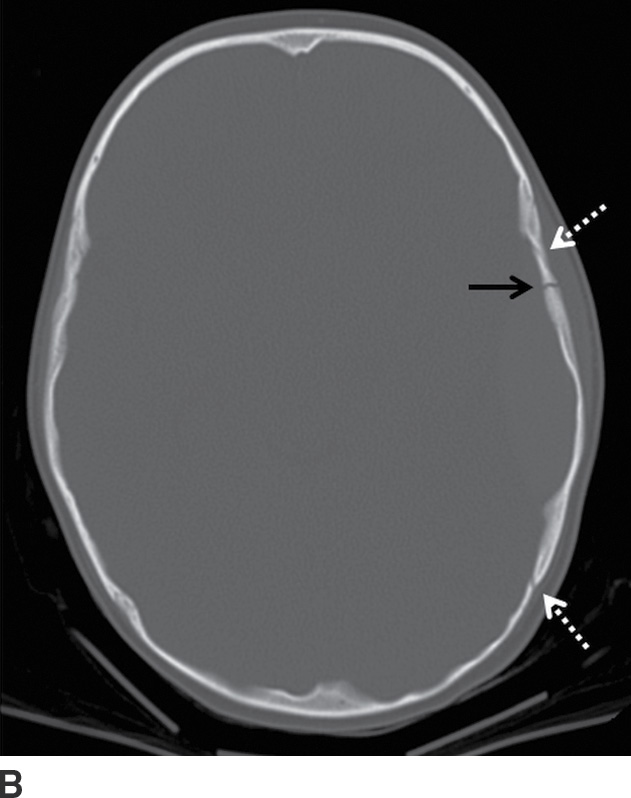
FIG. 3.2 Typical acute EDH. A: Axial CT in the brain window shows hyperdense lentiform/biconvex extra-axial hemorrhage (arrow). B: Corresponding image in the bone window shows overlying fracture of the adjacent squamous temporal bone (black arrow). The source of bleeding is likely from a tear of the adjacent middle meningeal artery. Note the EDH does not extend beyond the dural attachments at the coronal and lambdoid sutures (white arrows).
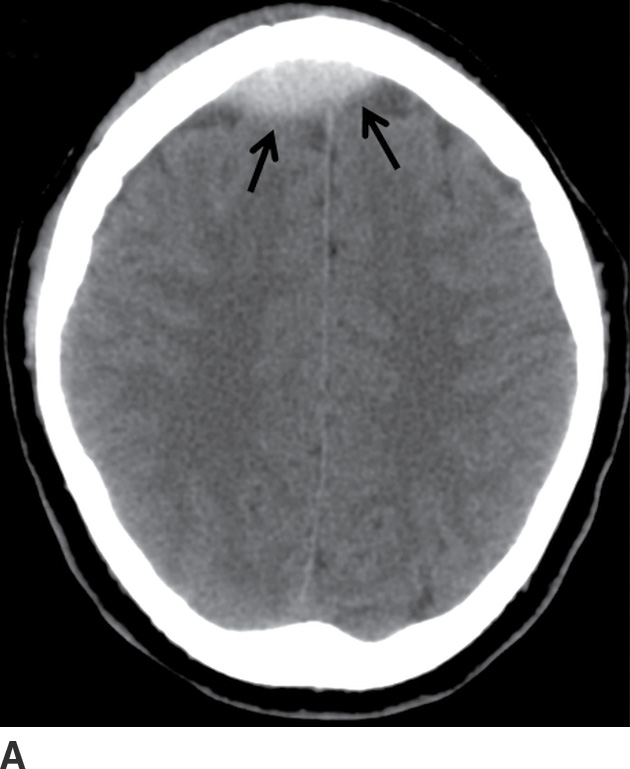
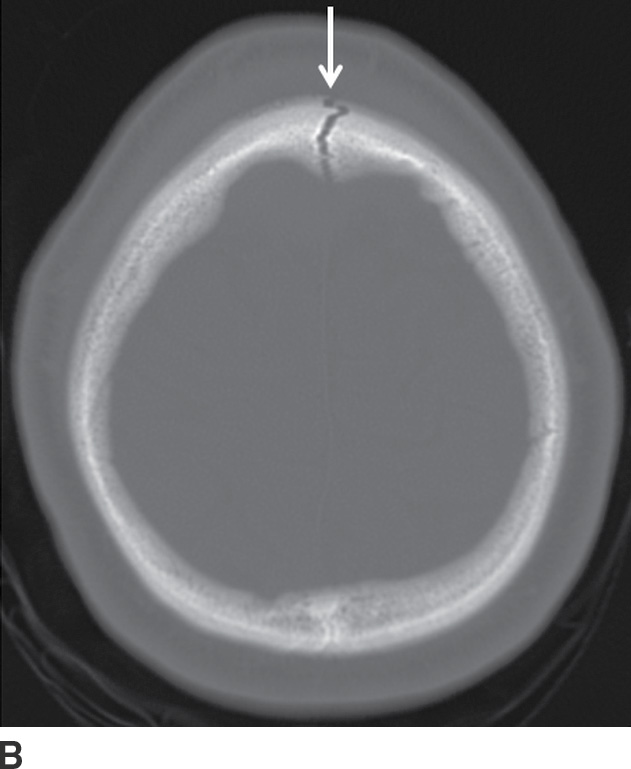
FIG. 3.3 Acute frontal EDH. A: Axial CT in the brain window shows lentiform hemorrhage that crosses midline, anterior to the falx (arrows). B: Slightly higher image in the bone window shows diastatic fracture through the sagittal suture (arrow). The source of bleeding is likely from a tear of the underlying sagittal dural venous sinus.
Subdural hematomas
An SDH collects between the inner layer of the dura and the arachnoid. The typical acute SDH usually occurs in infants, individuals who suffer from chronic alcoholism, or the elderly, partly due to greater space between the brain and skull, and often compounded by frequent falls. The bleeding source is often from tearing of bridging cortical veins that cross the subdural space to enter a dural venous sinus. Less commonly, SDH can also occur from tearing of cortical arteries, if associated with a skull fracture. Unlike EDH, SDH can occur from relatively minor head trauma, often without associated fractures. They can also occur without a confirmed history of trauma, particularly in patients who are anticoagulated or have other increased risks of bleeding.
SDHs are usually located over the cerebral convexities, are crescentic or curvilinear in shape, and are mostly limited by dural reflections, but not by sutures (Fig. 3.4). Therefore, they can be quite elongated in the anteroposterior dimension. SDH can also occur in the interhemispheric fissure, along the falx. Subdural blood often collects along the tentorial membrane, giving a more pronounced hyperdense outline of the tentorial incisura. Occasionally, subdural blood along the tentorium can be large and asymmetric (Fig. 3.5). Posteriorly, subdural blood can outline the superior sagittal sinus, resulting in a “pseudodelta” appearance (Fig. 3.6). Rarely, SDH can also be intrafalcine, which gives a biconvex midline shape. The prognosis of patients with SDH can be worse than those with evacuated EDH, even after SDH evacuation. Relatively small SDHs can also be clinically deceiving in children and young adults, because they generally have less space in the cranial compartment to accommodate even a small collection. In this population, this can also be associated with unusually severe brain edema, and aggressive surgical management with preemptive decompressive craniectomy is often performed in these individuals (Fig. 3.7). Although uncommon, rapid enlargement of an acute SDH is unpredictable, and repeat CT may be warranted to ensure stability (Fig. 3.8), even without clinical deterioration.
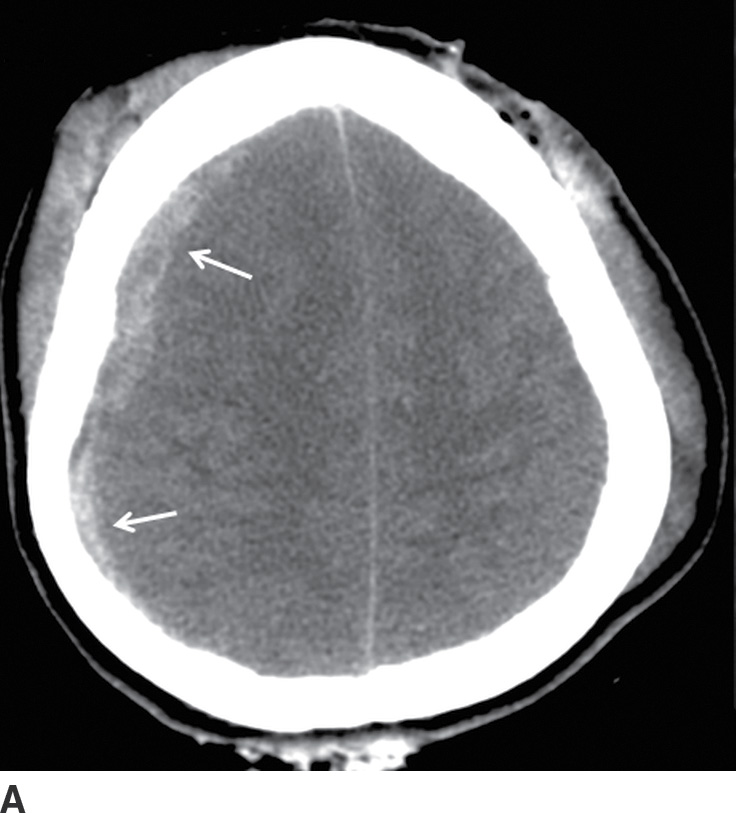
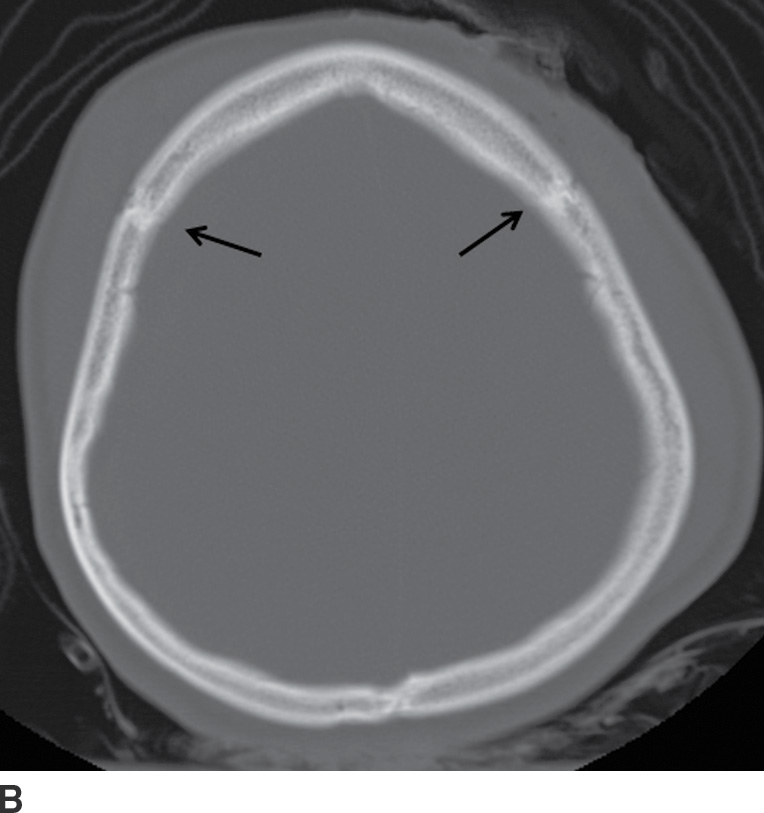
FIG. 3.4 Typical acute SDH. A: Axial CT in the brain window shows crescentic extra-axial hemorrhage (arrows) that extends over right frontal and parietal convexity. B: Corresponding image in bone window shows the location of the coronal sutures (arrows). Note the SDH is not limited by the sutures.
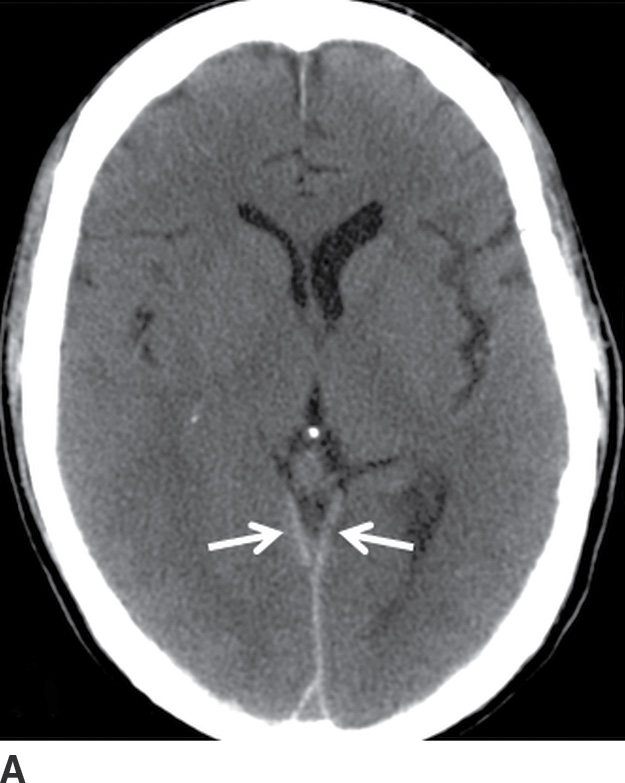
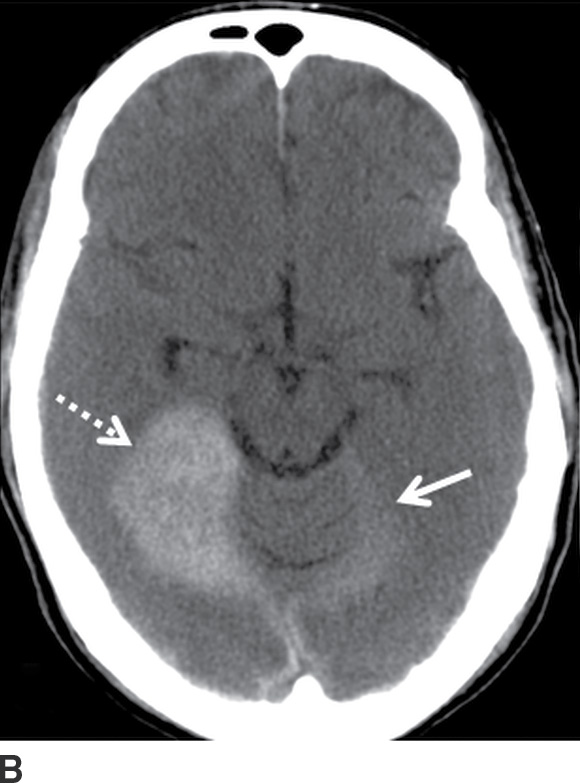
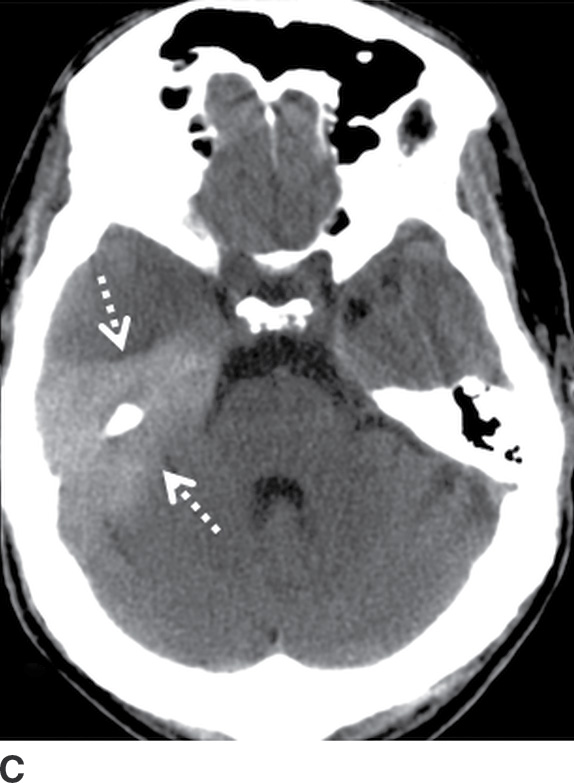
FIG. 3.5 Atypical peritentorial SDH. Axial CT images (A-C) from a TBI patient with SDH that extends along the tentorium (solid arrows), with a larger right-sided component that protrudes into the right middle cranial fossa and anterior right side of the posterior fossa (dotted arrows).
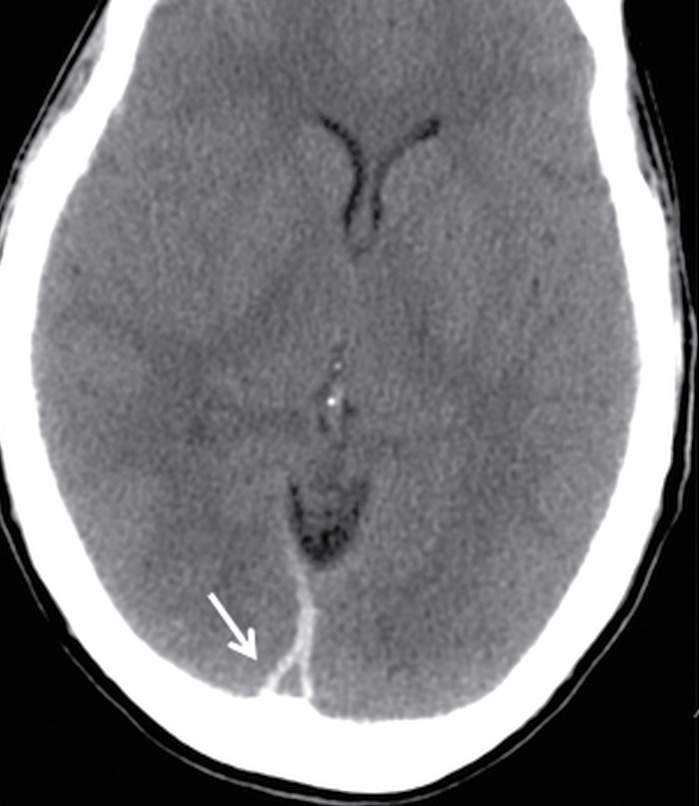
FIG. 3.6 Pseudodelta sign of SDH. Axial CT from a TBI patient shows the triangular appearance of hyperdense subdural blood bordering the posterior aspect of the superior sagittal sinus (arrow). This resembles the delta sign that occurs in contrast-enhanced scans when there is thrombus within the central lumen of the superior sagittal sinus, surrounded by a margin of enhancing blood or dura.
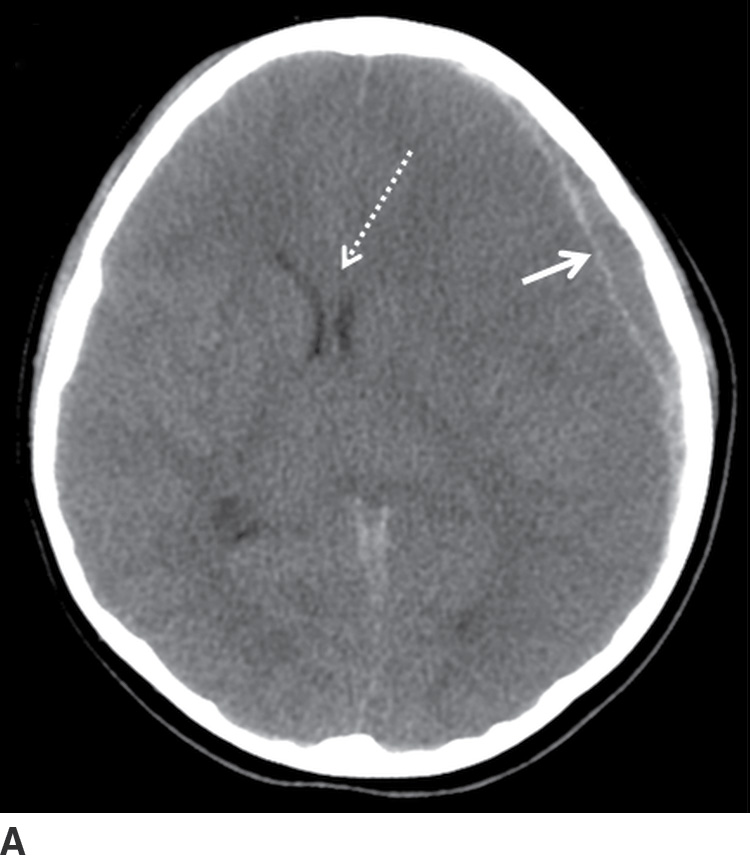
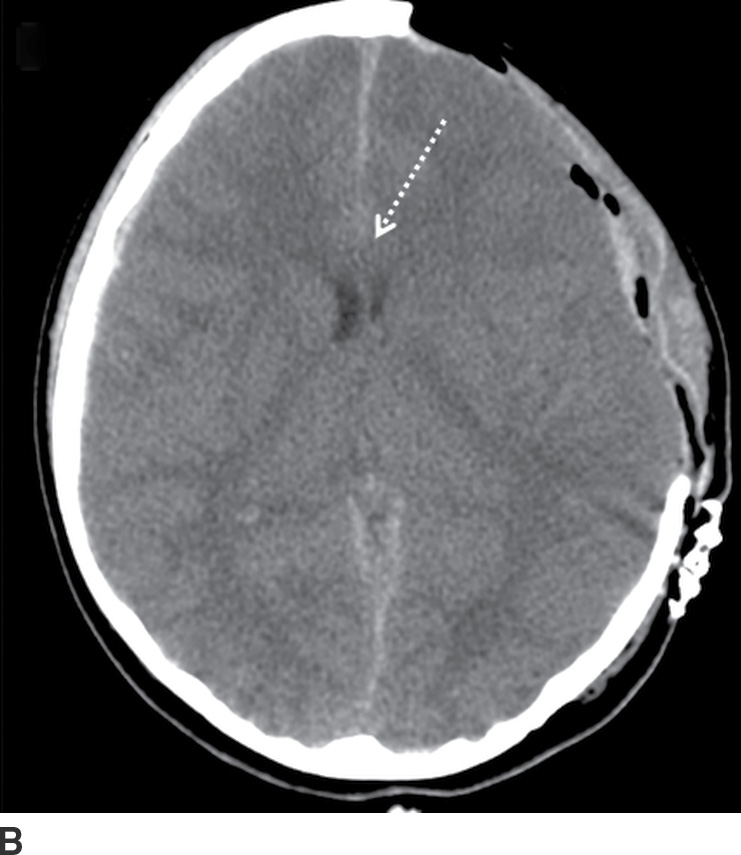
FIG. 3.7 Acute SDH with decompressive craniectomy. A: Axial CT in a 6-year-old child who was struck by a car shows relatively small, but predominantly hypodense, SDH (solid arrow), with significant mass effect and midline shift (dotted arrow). B: She underwent immediate decompressive craniectomy, with improved midline shift (dotted arrow).
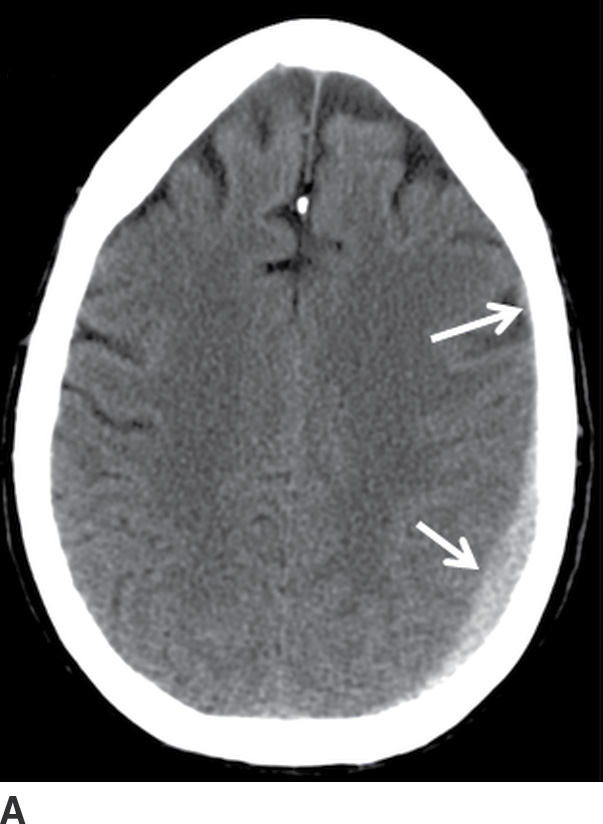
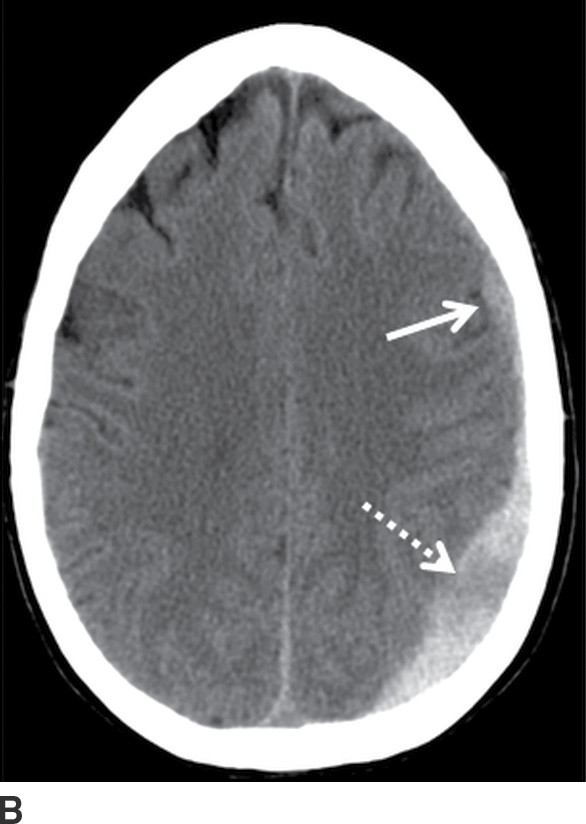
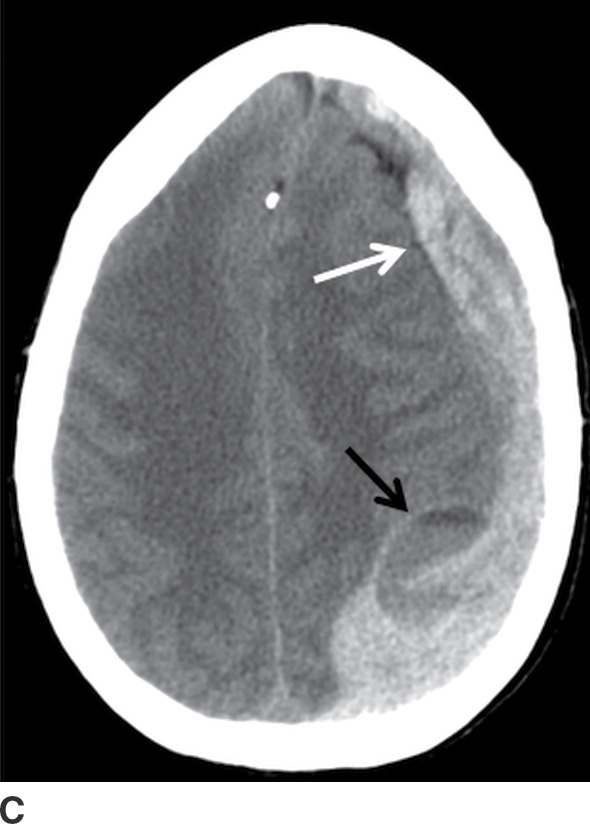
FIG. 3.8 Rapidly enlarging SDH. Serial axial CT images within a 24-hour period, in a patient involved in a motor vehicle accident. A: Initial scan shortly after hospital admission showed a small crescentic SDH (arrows). B: An 8-hour follow-up CT was ordered while the patient was in the intensive care unit but still neurologically intact, which showed moderate enlargement of the SDH (solid arrow) and focal hypodense area (dotted arrow) concerning for active bleeding. C: After neurologic deterioration, another CT obtained 20 hours after the first scan showed marked progression (white arrow) with very heterogeneous areas including a loculated component with fluid–fluid level (black arrow) at the prior hypodense site.
The terms acute, subacute, and chronic are often used to describe the evolution of SDH, although there is little consensus on their definitions. The terms are useful for general descriptive purposes but should be used with caution in the setting of possible nonaccidental trauma (NAT), as there are potential legal implications and debates that may arise over terminology in radiology reports. Typically, SDH tends to become isodense within several days (Fig. 3.9), although there is significant variability in evolution, and some patients can continue to have hyperdense blood for more than a week. Uncomplicated chronic SDH will become similar to CSF in density but will usually have subtle heterogeneity. MRI will often show that the signal is not identical to CSF. In addition to subacute blood, diffusely isodense SDH can be due to anemia, coagulopathy, or CSF (from torn arachnoid) mixed with blood. Similarly, hypodense SDH is not always due to chronicity and can be due to coagulopathy, anemia, or dilution with CSF leaking into the subdural space from a tear in the arachnoid.
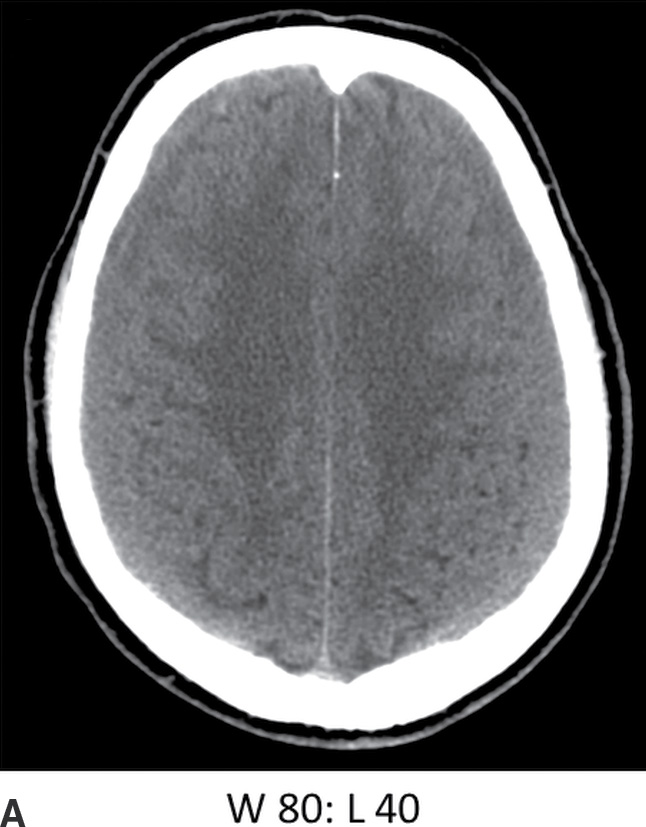
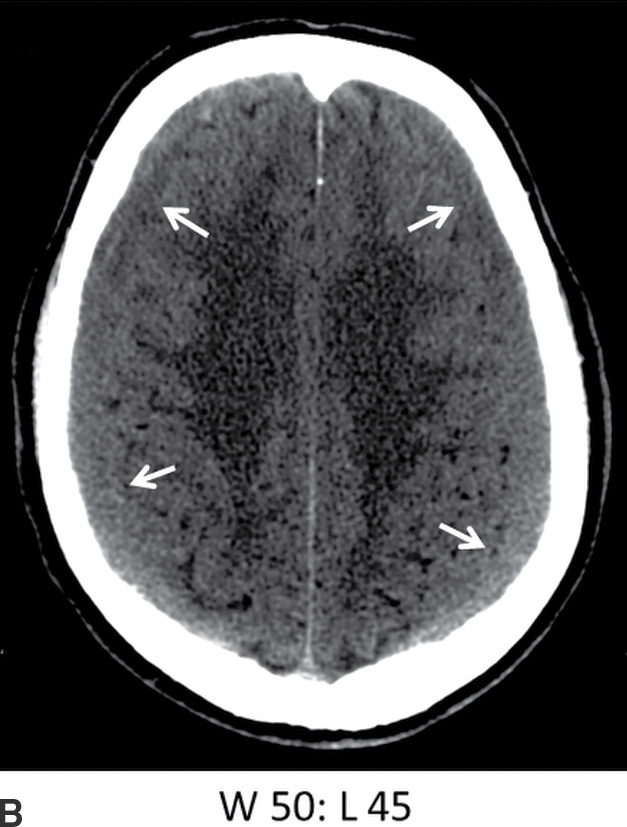
FIG. 3.9 Isodense SDH. A: Axial CT in the conventional brain window illustrates the difficulty in detecting isodense bilateral SDH. B: Changing the window to a narrower width/level can improve recognition of isodense SDH (arrows) by accentuating slight differences in density as well as the surface sulci. Note the white matter hypodensity is also more pronounced, which can be helpful in detecting unilateral isodense SDH, as the white matter margins will be noticeably asymmetric.
The presence of heterogeneity with hypodense areas can also occur for several different reasons and should not be automatically interpreted as “acute (hyperdense) on chronic (hypodense)” hemorrhage. Like EDH, focal hypodense areas could indicate active bleeding but can also be due to coagulopathy or admixture with CSF. If there is a single transverse demarcation (on axial images) with a hypodense upper component and hyperdense lower component, this indicates sedimentation or a “hematocrit” effect, which can occur relatively quickly in patients with clotting disorders or can occur with acute blood settling below older blood (Fig. 3.10). Hypodense CSF can also be mixed with acute SDH if there is a concomitant tear of the arachnoid. If there is a vertical demarcation (on axial images) with a hypodense inner/medial component and a hyperdense outer/lateral component, this may be due to an enlarged subarachnoid space next to the acute SDH, which can occur in infants and which should not be mistaken for “blood of different ages.”
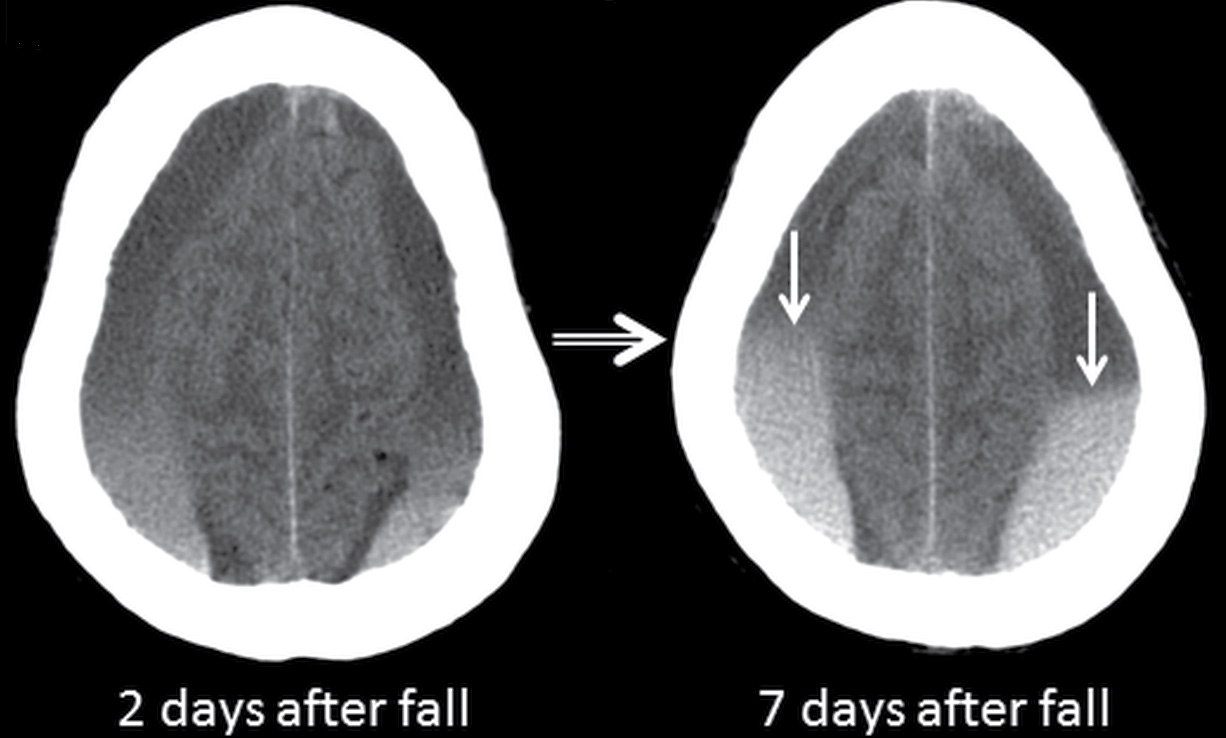
FIG. 3.10 Sedimentation in SDH. Axial CT images in an elderly patient on anticoagulation therapy who fell after a syncopal episode, 2 days prior to admission. Bilateral SDHs are present, with sedimentation or hematocrit effect, due to dependent layering of heavier hyperdense red blood cells. The first scan shows a gradual gradation of hypodense serous component superiorly to hyperdense red blood cells inferiorly. Over the course of 5 days, the dependent portion is larger and more dense, with more sharply defined layer (arrows) as the clot develops.
Components of subacute or chronic SDH can be diagnosed with greater certainty if there are internal septations or “neomembranes” (Fig. 3.11). MRI can be helpful to better delineate extra-axial collections (Fig. 3.12). FLAIR or intermediate-weighted MRI sequences are more useful and show higher signal than normal CSF. SWI may show patchy areas of very hypointense signal, but the absence of such signal does not exclude the presence of blood products. Small hypointense convexity SDHs can be harder to delineate on SWI due to the proximity to hypointense bone. However, subdural blood along the tentorium is often detectable as irregular hypointensity on this sequence. DWI can also sometimes show diffusion restriction in the presence of hemorrhage, which should not be mistaken for purulent material (Fig. 3.13).
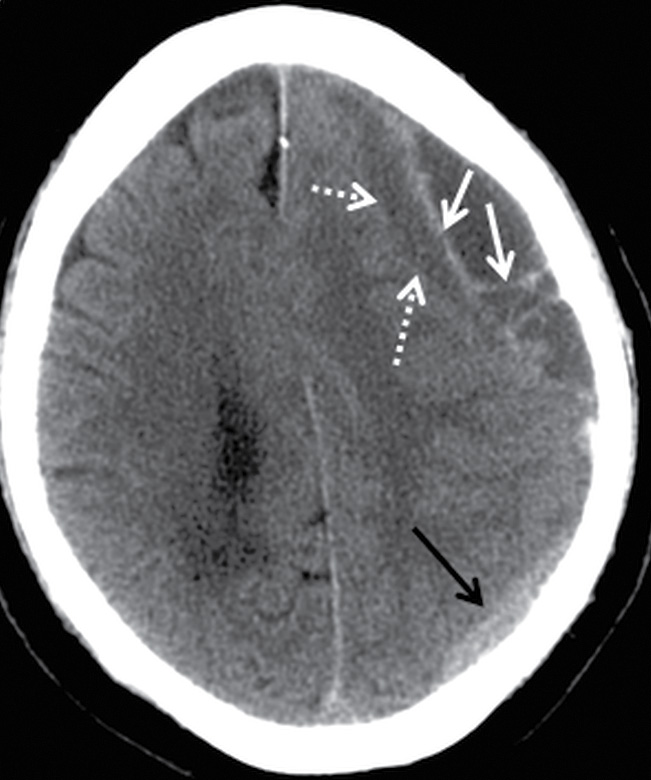
FIG. 3.11 Acute-on-chronic SDH. Axial CT image from an elderly man who fell and struck his head 1 month before hospital admission and subsequently fell several more times before presenting with 1-week history of confusion. There is a heterogeneous extra-axial collection over the left hemispheric convexity. The larger anterior component has several loculated older hypodense compartments with acute hyperdense blood outlining internal septations and membranes (white arrows). The underlying surface of the brain (dotted arrows) is compressed. Another smaller hyperdense component is seen more posteriorly (black arrows). Note that the acute-on-chronic SDH has a lentiform/biconvex shape, compared to the typical crescentic morphology of the acute SDH posteriorly.
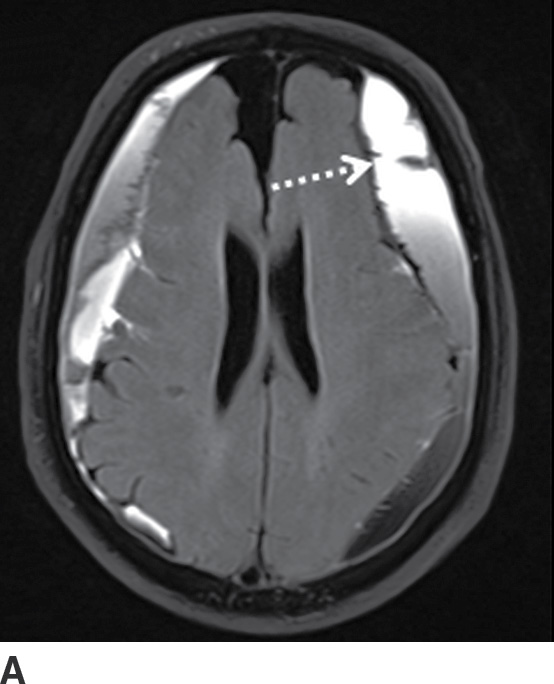
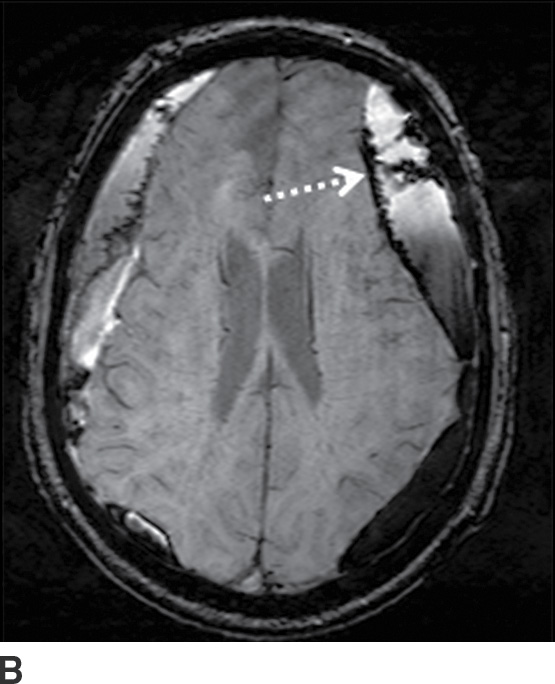
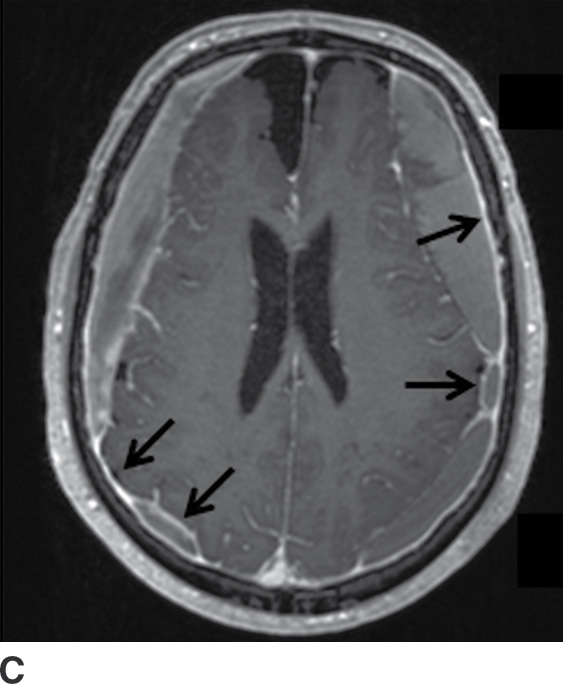
FIG. 3.12 Evolution of SDH on MRI. An elderly man on anticoagulation therapy had sudden onset of neurologic symptoms, initially suggestive of an acute stroke. MRIs obtained 10 days after presentation demonstrate characteristics of an evolving SDH, with internal loculation and heterogeneous signal characteristics (dotted arrows) on FLAIR (A) and SWI (B), as well as enhancing dura and membranes (black arrows) on postcontrast T1WI (C).
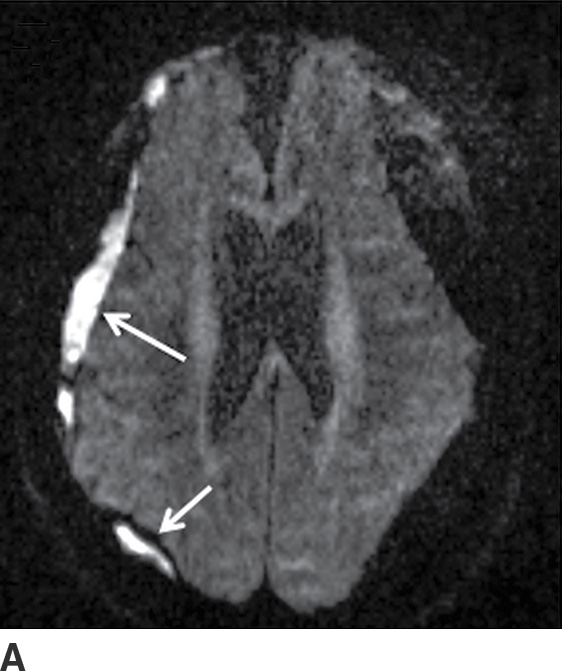
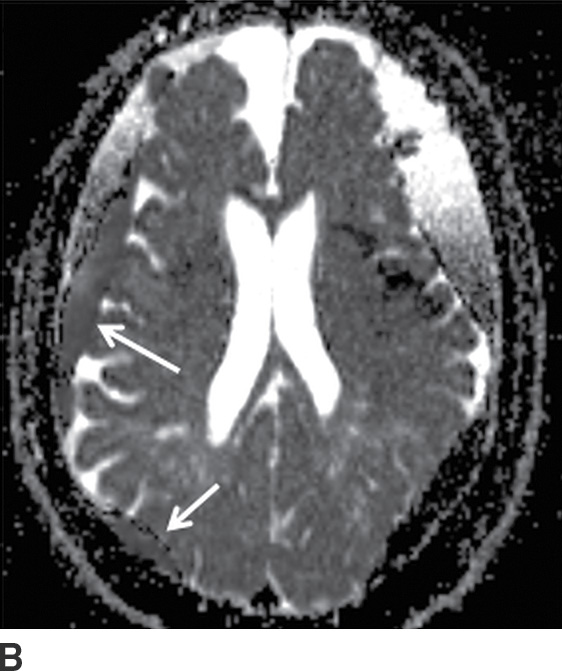
FIG. 3.13 Diffusion restriction in SDH. Axial MRI in same patient as in Figure 3.12. Bilateral heterogenous subdural collections are seen. Smaller internal loculations are present on the right, which demonstrates diffusion restriction (solid white arrows) on diffusion image (A) and corresponding ADC map (B).
Diffusion Restriction in SDH
Nonhemorrhagic traumatic subdural collections include subdural hygromas, which have also been referred to as “subdural hydromas” and “external hydrocephalus.” First described in the 1920s, these subacute or chronic CSF collections (with absence of, or minimal hemorrhage) are caused by a tear in the arachnoid, allowing CSF to leak into the subdural space (14). The term “subdural hygroma” was later coined for this entity but is sometimes incorrectly used for chronic SDHs, as well as any hypodense extra-axial collection in children. These traumatic collections are probably better referred to as “traumatic subdural effusions,” although the term “effusion” can be confusing because it is also used to describe meningitis-related collections. Perhaps the phrase “post-traumatic CSF collections in the subdural space” is more precise. Regardless, these collections may enlarge slowly after head trauma and are often unrecognized unless there are serial scans and occasionally can cause significant mass effect (15) (Fig. 3.14).
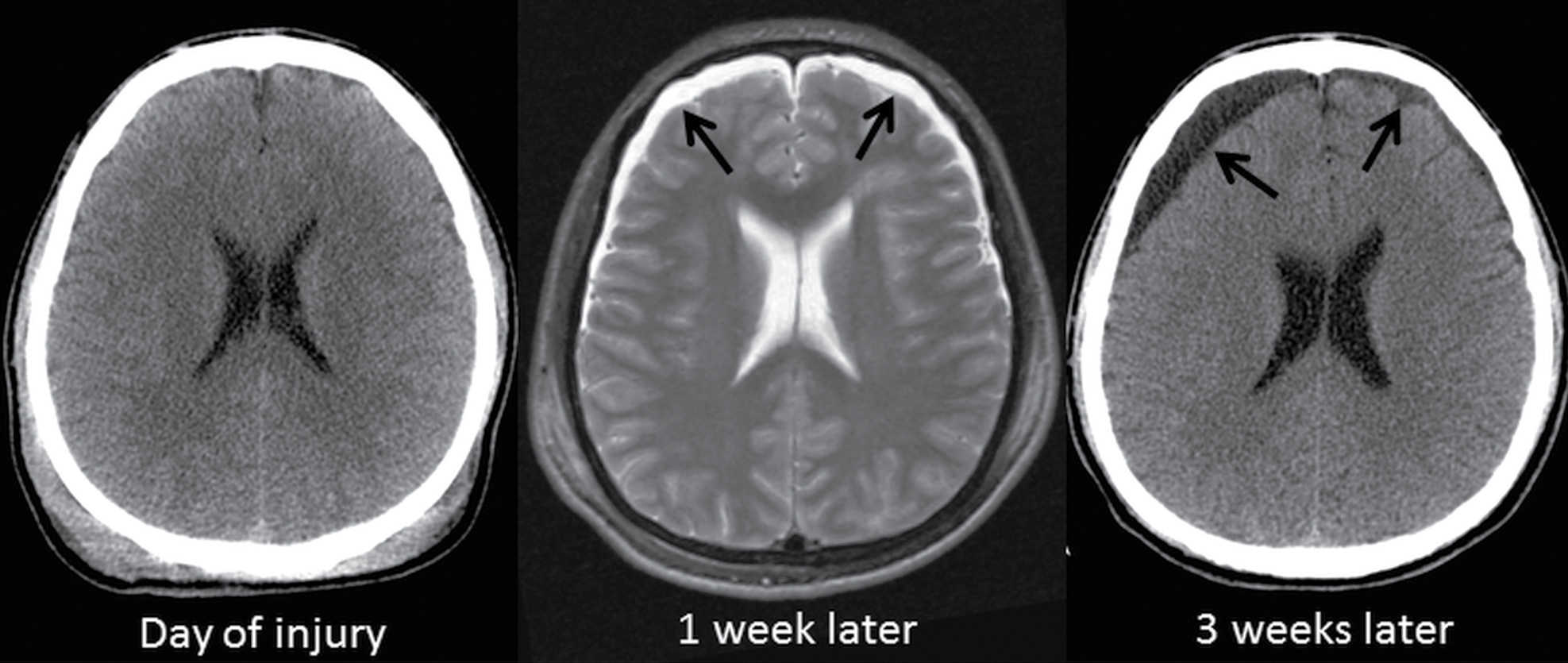
FIG. 3.14 Subdural hygroma. Axial images in at TBI patient ejected from a motor vehicle shows progressive enlargement of asymmetric CSF-like collections (arrows) over the frontal lobes over the course of 3 weeks.
Traumatic subarachnoid hemorrhage occurs between the arachnoid and pia, often within sulci and cisterns. Trauma is the most common cause of SAH, and tSAH is the most common type of extra-axial hemorrhage after head injury. It often occurs in conjunction with EDH or SDH. Traumatic SAH can occur from tearing of cortical arteries or veins, hemorrhagic contusions leaking through damaged pia, or spread from intraventricular hemorrhage. The location of tSAH can be in sulci or sylvian fissures from coup or contrecoup injury, or in basal cisterns (Fig. 3.15). A commonly overlooked location is the interpeduncular cistern, which may be the only clue to the presence of tSAH on CT. This can also be a marker of brainstem injury, often in the setting of diffuse axonal injury (DAI). The presence of tSAH is not consistently associated with outcomes, although some investigators have shown a relationship between interpeduncular tSAH and brainstem injuries, as well as a correlation between thickness of visible SAH and worse outcome (16).
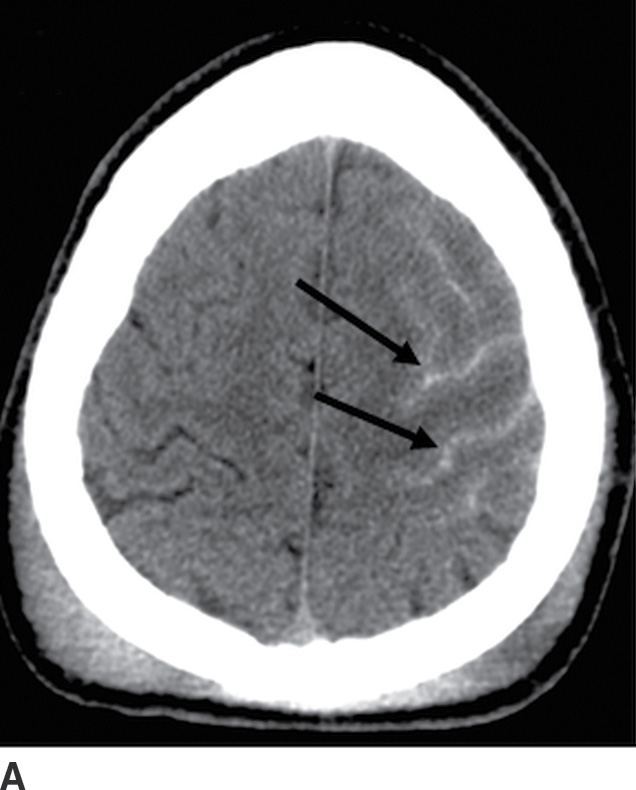
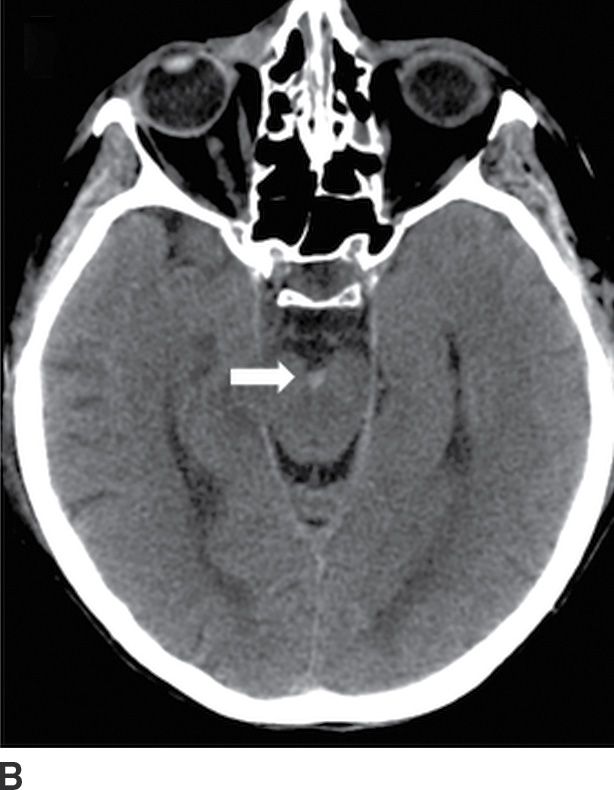
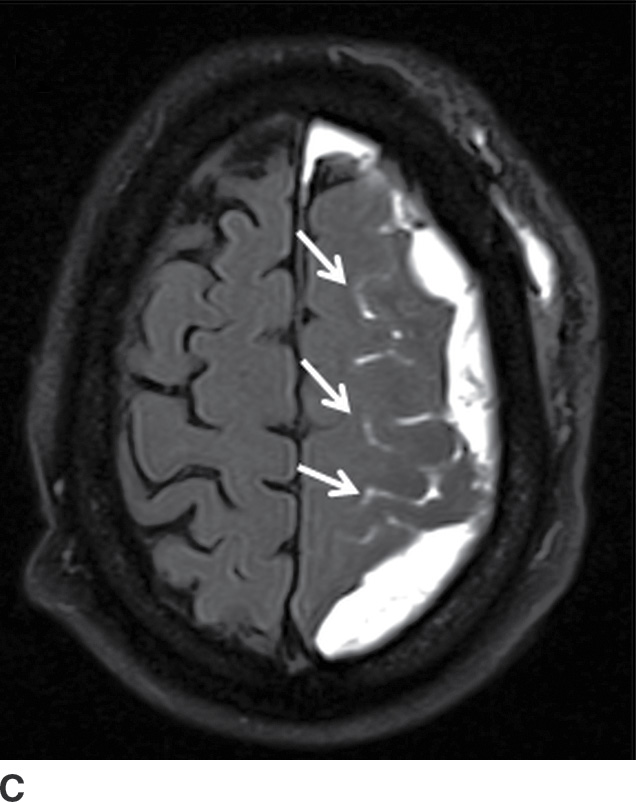
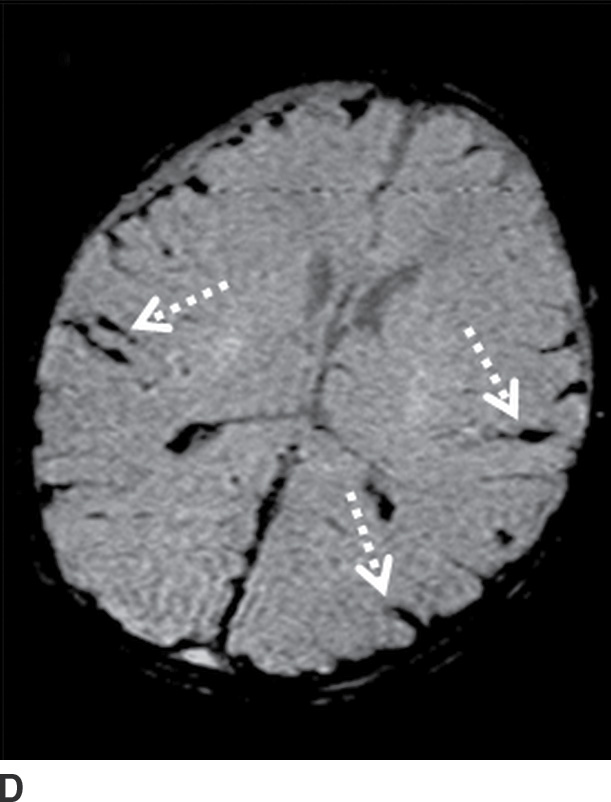
FIG. 3.15 Traumatic subarachnoid hemorrhage. Images from four different TBI patients. A: Traumatic subarachnoid hemorrhage (tSAH) is common in the peripheral sulci adjacent to a site of impact (arrows). A frequently overlooked site of tSAH is in the interpeduncular cistern (B, arrow), which may be the only clue to TBI. tSAH will be hyperintense on FLAIR (C, arrows) and can also be visible on SWI (D, dotted arrows).
tSAH over the convexities appears as small curvilinear areas of hyperdensity within the sulci, while blood in the cisterns can be thicker, except for punctate hyperdensity in the interpeduncular cistern. tSAH in the paramedian sulci tends to be perpendicular to the falx, whereas small linear areas of hyperdense blood parallel to the falx are more likely subdural in location. Unlike SAH from aneurysm rupture, tSAH tends to be more peripheral, scattered, and patchy, rather than centered in a single cistern. tSAH can be visible on MRI, particularly FLAIR (hyperintense), but also on SWI (hypointense) (17). Be aware that hyperintense FLAIR signal within the sulci can also occur with meningitis, or if the patient is given 100% oxygen during general anesthesia, which should not be mistaken for SAH. Occasionally, tSAH can persist into a chronic phase and develop superficial siderosis, which may be evident as subtle hyperdensity outlining the margins of the cortex or brainstem on CT or as hypointense signal outlining the brain on SWI (and to a lesser degree on conventional T2*-GRE or T2WI).
Traumatic intraventricular hemorrhage
A traumatic intravertebral hemorrhage is relatively uncommon in TBI but can occur from tearing of subependymal veins during shearing forces (Fig. 3.16). Although it is associated with poor outcomes, this seems to be primarily due to other injuries (18).
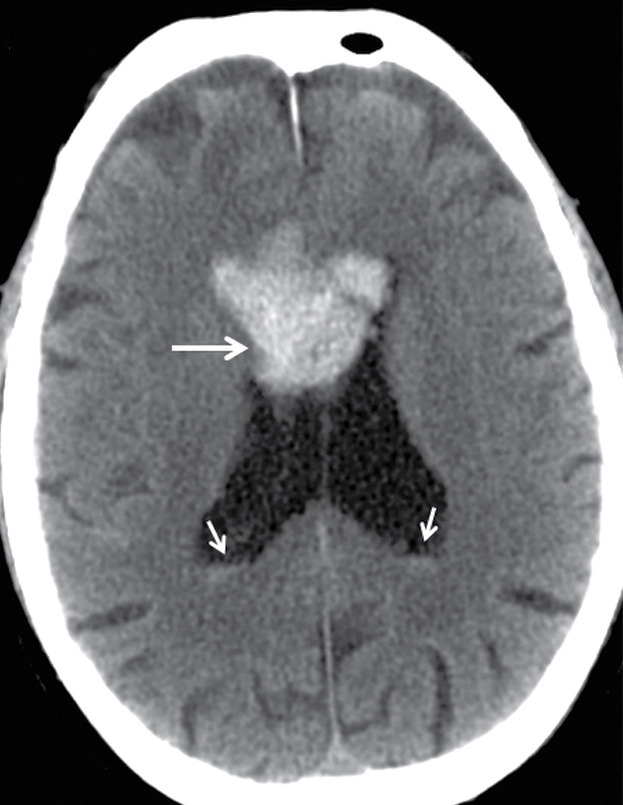
FIG. 3.16 Traumatic intraventricular hemorrhage. Axial CT image at the level of the lateral ventricles from an elderly man who fell shows a midline intraventricular hemorrhage (long arrow) that is likely due to bleeding from subependymal veins. Small amounts of blood are layering in the occipital horns (short arrows).
Parenchymal contusions
Parenchymal contusions typically result from focal injury and can be named according to etiology (Table 3.7). They can be coup or contrecoup and can be hemorrhagic or nonhemorrhagic. Contusions tend to be located along the periphery of the brain and are usually due to impact against an adjacent structure that is more rigid—such as the calvarium, internal bony ridges (particularly the irregular floor of the anterior cranial fossa or sharp edges of the lesser sphenoid wings), falx, or tentorium. Any abrupt force or rapid acceleration/deceleration to the head will allow the brain to bounce against these rigid structures. In general, the most common locations are the inferior frontal gyri (Fig. 3.17) and the anterior temporal poles (Fig. 3.18). The lateral temporal gyri and anterior frontal gyri are also frequently involved. If there are focal impact injuries, the underlying gyri in these regions are also likely affected. Other locations include the posterior body or splenium of the corpus callosum (Fig. 3.19) and the midbrain. However, in both of these latter locations, it can be difficult to determine if the injuries are due to contusion, shearing, or other mechanisms.
Table 3.7 CONTUSIONS
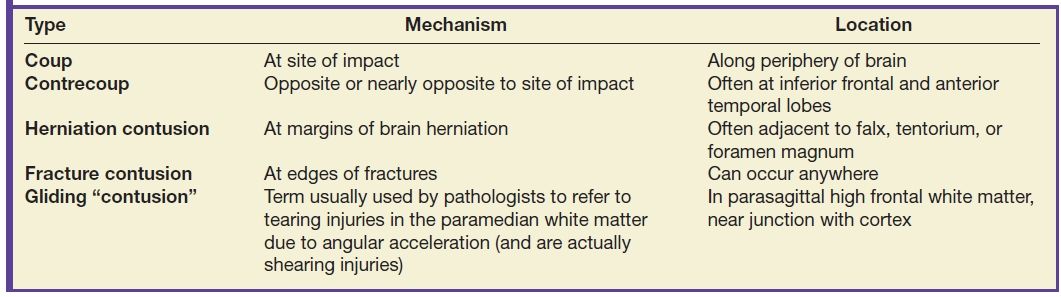
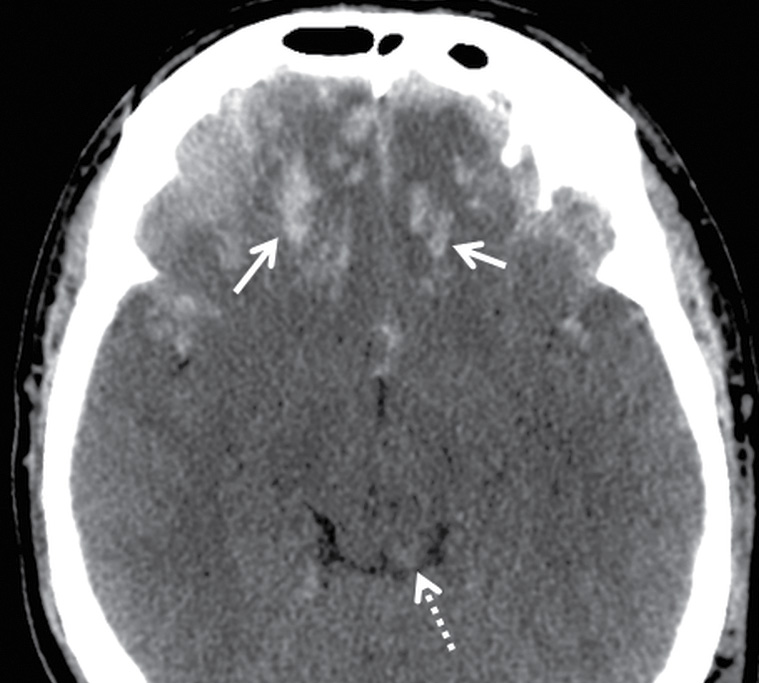
FIG. 3.17 Inferior frontal contusions. Axial CT image from an adult assault victim showing numerous small contusive hemorrhages in the bilateral inferior frontal lobes (solid arrows), which is a common location for contusion, as the brain slides back and forth over the irregular contour of the floor of the anterior cranial fossa. There is also hemorrhage in the dorsal midbrain (dotted arrow), which could be due to contusion or shearing injury.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree