Fig. 1
Synchronous Krukenberg tumours of the ovaries in a 60-year-old woman who presented with abdominal distension. (a) Axial contrast-enhanced CT image demonstrates bilateral, bosselated, predominantly solid ovarian masses (arrows) with multiple minor cystic components and a heterogeneous enhancement pattern. A small amount of ascites is present (asterisks). Axial T1-weighted Volumetric Interpolated Breath-hold Examination (VIBE) images with fat-saturation pre- (b), 30 s (c) and 180 s (d) post-gadolinium injection demonstrate avid early heterogeneous enhancement of the right adnexal tumour, which persists in the delayed phase (arrows). The uterus is also seen (curved arrows)
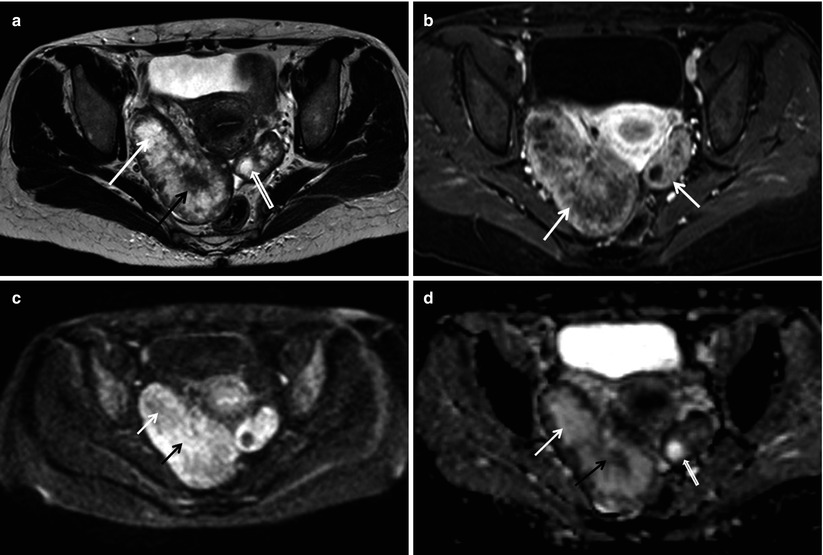
Fig. 2
Synchronous Krukenberg tumours of the ovaries in a 42-year-old woman with no prior history of malignancy, who presented with vaginal bleeding. Upper GI endoscopy, performed after imaging suggested the probability of ovarian metastases, diagnosed primary gastric cancer (diffuse adenocarcinoma with signet-ring cells). (a) Axial T2-weighted fast spin echo (FSE) MR image shows bilateral heterogeneous, predominantly solid adnexal masses, surrounding by a thin hypointense capsule, with areas of hyperintensity (white arrow), attributed to mucin/oedema, and scattered diffuse or linear relatively hypointense components (black arrow), representing fibrosis. A more well-defined intratumoural cyst is noted (open arrow). (b) Gadolinium-enhanced axial T1-weighted spin echo image with fat suppression demonstrates dense enhancement of the capsule and solid components of the lesions (arrows). (c) High b-value (1,000 smm−2) axial diffusion-weighted image (DWI) shows increased signal intensity of the solid components (black arrow) and intermediate of the mucinous components (white arrow). (d) Corresponding mono-exponential apparent diffusion coefficient (ADC) map from b-values of 0, 100, 250, 500 and 1,000 smm−2 confirms restricted diffusion of the solid components (black arrow) and intermediate diffusion of the mucinous areas (white arrow). The intratumoural cyst shows free diffusion (open arrow)
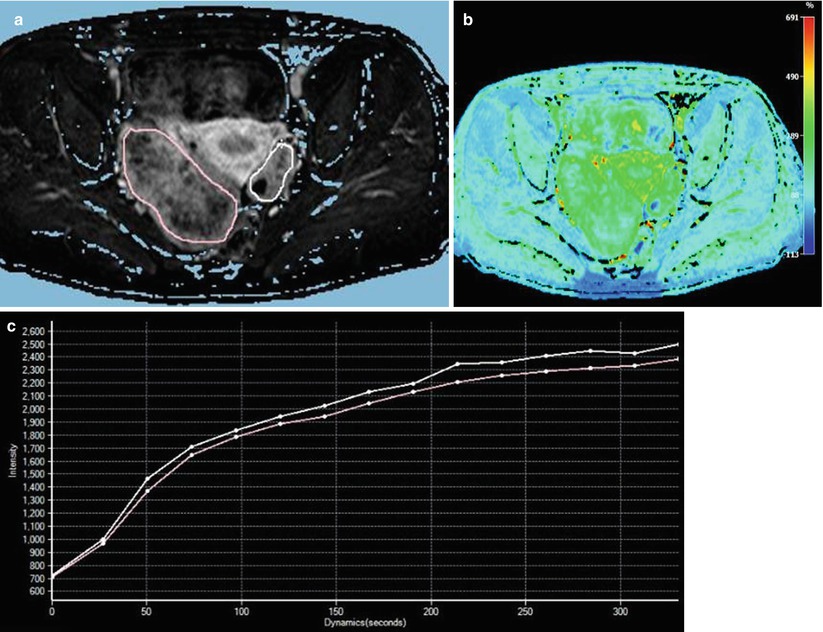
Fig. 3
Perfusion MR study of Krukenberg tumours of the ovaries in a 42-year-old woman with primary gastric adenocarcinoma (same patient as in Fig. 2). (a) Axial T1-weighted High Resolution Isotropic Volume Excitation (THRIVE) image with fat suppression after bolus administration of gadolinium chelate at a dose of 0.2 ml/kg body weight shows manually delineated regions of interest (ROI) encompassing the entire lesion area. Images were obtained sequentially at approximately 25 s for 5 min. (b) Colour-coded map of relative enhancement demonstrates moderate enhancement in the solid portions of both tumours. (c) Graph shows the signal intensity-time curve, characterised by early onset, high amplitude and delayed persistence of enhancement, indicative of an aggressive tumour behaviour
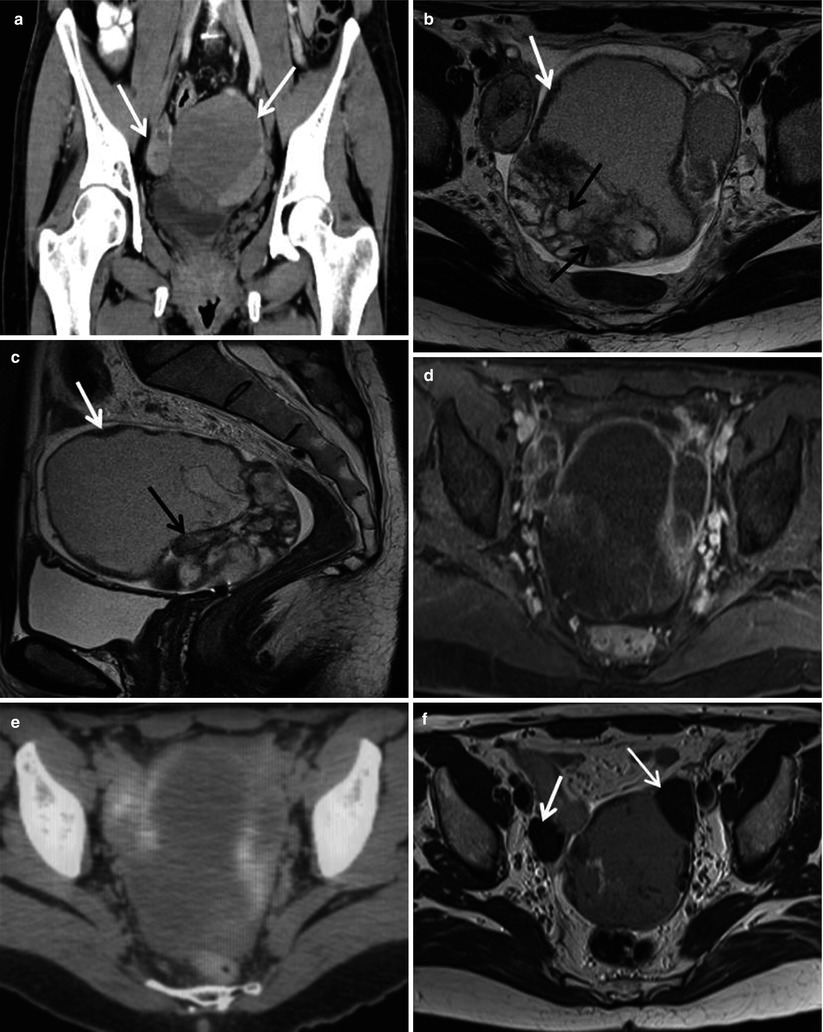
Fig. 4
Synchronous Krukenberg tumours from a moderately differentiated adenocarcinoma of the proximal rectum in a 48-year-old woman. (a) Coronal contrast-enhanced CT image demonstrates bilateral complex adnexal masses (arrows). On axial (b) and sagittal (c) T2-weighted FSE images, the bulky left ovarian tumour has dominant well-defined cystic components of varying mucin concentration surrounded by a thin hypointense rim (white arrows). In the more solid posterior portion of the tumour, abundant nodular and linear/branching hypointense septa are seen (black arrows). (d) Axial T1-weighted VIBE fat-saturated image demonstrates irregular and nodular enhancement of septa and rim. (e) Fused axial FDG-PET image shows low to moderate, predominantly peripheral metabolic activity. (f) Post-chemotherapy axial T2-weighted FSE image demonstrates reduction in size of the adnexal masses and development of new T2-hypointense areas (arrows), possibly due to a fibrotic response
Metastatic Mucinous Adenocarcinomas (Non-Krukenberg Type)
The majority of mucinous ovarian adenocarcinomas are metastatic; in a series of 52 mucinous ovarian tumours, 40 (77 %) were secondary deposits, originating in descending order of frequency from the gastrointestinal tract (35 %), pancreas (15 %), uterine cervix (10 %), breast (6 %) and endometrium (4 %), while in 7 % the primary site could not be identified [81]. Bilateral distribution and tumour size <10 cm were strongly associated with metastases (respective frequencies 77–94 % and 87–95 %), inasmuch as the criterion of a unilateral lesion larger than >10 cm could predict a primary tumour with 84–90 % accuracy [81, 82]. In a retrospective study of 194 mucinous tumours, performance of the algorithm was optimised at a size cut-off value of 13 cm, with correct classification of 98 % of primary tumours and 82 % of metastases (87 % overall accuracy) [83].
Mucinous metastases mainly originate from the uterine cervix (particularly adenocarcinomas) [28, 29, 84, 85], colon [86], pancreas [87, 88], gallbladder [73, 89] and appendix [90] and need to be differentiated from primary mucinous tumours of the ovaries. The most pertinent morphological and clinical criteria include localisation of mucin (intra- vs. extracellular), laterality and size of the lesion, presence of peritoneal dissemination and metastatic growth pattern (defined by ovarian surface involvement, lymphovascular invasion and desmoplastic response) [91]. For example, predominantly extracellular distribution of mucin, designating a colloid subtype of tumour, with concomitant peritoneal disease, defines pseudomyxoma peritonei, which most frequently arises from appendiceal low-grade or colloid mucinous carcinomas [92]. The term pseudomyxoma ovarii is used to describe extracellular mucin in tumour confined to the ovary and is frequently associated with ovarian mature teratomas [93]. Unilateral cystic carcinomas with predominantly intracellular deposition of mucin (greater than 50 % in at least 90 % of tumour cells) are the hallmark of primary mucinous tumours. Invasive mucinous carcinomas are distinguished from their low-grade counterparts by the presence of focal stromal invasion greater than 5 mm or an expansile growth pattern with lack of intervening stroma. Presence of peritoneal spread directs to a high probability of a metastatic origin of cystic tumours with intracellular mucin. In a histopathological study of 43 metastatic and 25 stage I primary mucinous tumours, morphological features indicating a metastatic vs. primary origin were bilaterality (75 % vs. 0 %, p < 0.0001), microscopic surface involvement (79 % vs. 0 %, p < 0.0001), an infiltrative invasive pattern (usually with desmoplastic stroma) (91 % vs. 16 %, p < 0.0001), nodular growth pattern (42 % vs. 0 %, p = 0.0003) and hilar involvement (31 % vs. 4 %, p = 0.0105) [94]. Findings associated with primary tumours were size > 10 cm (88 % vs. 48 %, p = 0.007), a smooth surface (80 % vs. 38 %, p = 0.005), expansile invasive pattern (usually with no intervening stroma) (88 % vs. 18 %, p < 0.0001), microscopic cysts < 2 mm (84 % vs. 40 %, p = 0.002) and benign- or borderline-appearing regions (57–76 % vs. 30–36 %, p ≤ 0.045). Macroscopic features which were not found discriminatory were a predominantly cystic or solid gross appearance and presence of focal papillary, necrotic or hemorrhagic areas [94].
On CT and MRI mucinous metastases from colorectal cancer typically manifest as unilocular or multilocular, predominantly cystic masses with various degrees of attenuation/signal intensity of the cystic components, reflecting the concentration of mucin and the extent of necrosis [68, 80]. Typically contrast enhancement is demonstrated by the internal septations and solid components [80]. In a study of 52 ovarian masses, smooth oval contours were reported in 92 % of metastases from colorectal cancer compared to 45 % of primary adnexal malignancies and were ascribed an odds ratio of 24.3 for the prediction of a metastatic aetiology [68]. Furthermore, a predominantly cystic appearance was documented in 86 % of metastases in contrast to 36 % of primary tumours (p = 0.005) [68] (Fig. 5). This finding contrasts the predominantly solid composition of Krukenberg tumours originating principally from gastric cancers [59, 61]. In this study other imaging features such as bilaterality, size, degree of enhancement of cyst walls and solid portions, volume of ascites and presence of peritoneal implants were not discriminatory between metastatic and primary tumours [68].
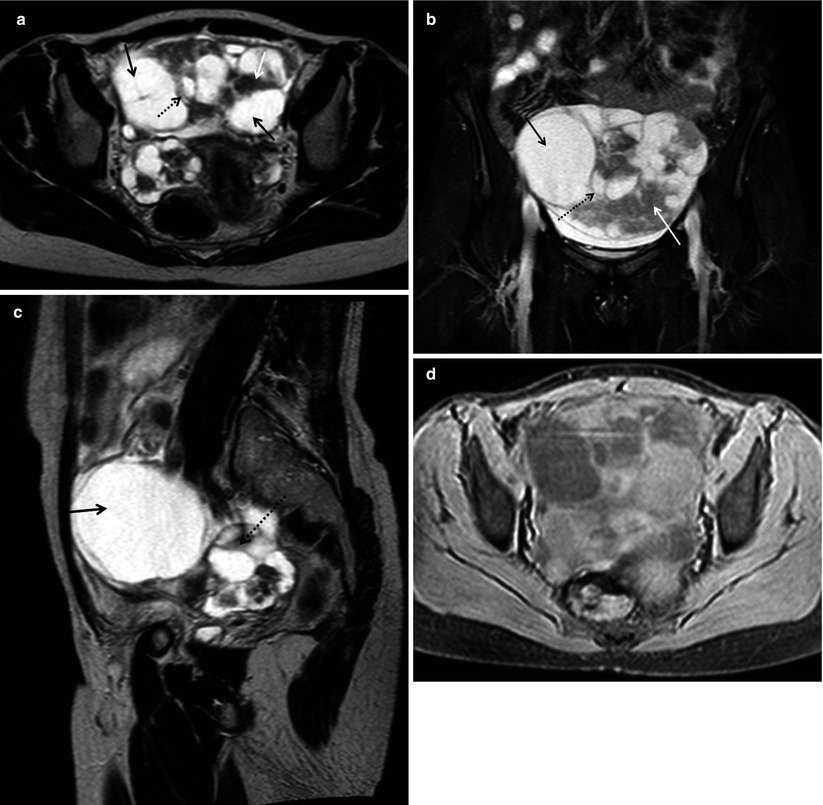

Fig. 5
Metachronous metastatic mucinous adnexal tumours in a 48-year-old woman with a history of primary colorectal adenocarcinoma (Dukes’ stage B) resected 18 months previously, who presented with abdominal distention. Axial (a), coronal (b) and sagittal (c) T2-weighted FSE images demonstrate bilateral multiloculated, predominantly cystic (black arrows) complex adnexal masses, with smooth borders and various conglomerates of solid mural nodules (white arrows) and thick septa (dotted arrows). (d) Axial gadolinium-enhanced T1-weighted image shows heterogeneous enhancement of the solid components. The imaging features are indistinguishable from a primary mucinous cystadenocarcinoma
Immunochemistry may be helpful in discriminating a primary ovarian mucinous tumour from metastatic colorectal adenocarcinoma. Positive staining for cytokeratins 7 and 20 (CK7+/CK20+) has been reported in 68–74 % of primary ovarian mucinous tumours, whereas the most common immunoprofile in lower intestinal tract tumours is CK7-/CK20+ (69–79 %) [95, 96]. In tumours with concomitant expression of CK7 and CK20, the pattern of immunostaining may be discriminatory; diffuse CK7 positivity, as defined by involvement of >50 % of tumour cells, with a focal or patchy (<50 % of tumour cells) CK20 distribution is frequently observed in primary ovarian tumours, whereas colorectal and appendiceal tumours typically display patchy CK7 and diffuse CK20 distribution [95, 96]. Loss of expression of Dpc4 tumour suppression gene has been reported of value in distinguishing metastatic pancreatic cancers from primary ovarian tumours, which often share the same pattern of CK7/CK20 positivity [88, 96]. The usefulness of other markers, such as the nuclear transcription factor CDX2 [97–102] and mucin core proteins MUC5AC and MUC2 [96, 103, 104], is limited by considerable overlap between primary and metastatic tumours.
Metastatic Endometrial Adenocarcinomas
Synchronous endometrial and ovarian carcinomas occur in approximately 9–12 % of patients with ovarian and 5 % of patients with endometrial carcinoma [25, 46, 105]. The designation of a dual primary versus metastases may be challenging, since 68–93 % of tumours detected concurrently in the endometrium and ovary are of endometrioid type, with or without squamous differentiation [25, 106, 107]. However, the similar histology may result from metastatic disease or reflect a common tumourigenic process independently affecting multiple tissues of common embryologic descent [108]. The distinction is critical, since standard treatment of endometrial carcinoma with metastatic adnexal involvement (stage IIIA) comprises surgery and adjuvant chemo- and/or radiotherapy, whereas independent stage I uterine and ovarian endometrioid carcinomas have a better overall prognosis and do not routinely require adjuvant treatment [25, 107, 109, 110]. In clinical stage I and II endometrial cancer, the incidence of ovarian metastases has been reported as 5–8 % [62, 111, 112].
Ulbright and Roth developed “empirical” pathological criteria for the characterisation of primary endometrial cancer with ovarian metastases, which comprise a micronodular ovarian pattern (major criterion) or two or more of the following: small (<5 cm) size of ovaries, bilateral involvement, deep myometrial invasion, vascular invasion and tubal lumen involvement (minor criteria) [113]. Scully et al. proposed a more extensive list of clinicopathological features to delineate metastatic disease, which additionally include the presence of atypical endometrial hyperplasia, absence of ovarian endometriosis, typical spread pattern of endometrial cancer, hilar location, vascular space invasion and surface implants in the ovarian site and similar molecular genetic and karyotypic abnormalities in both tumours [114]. Nevertheless, these criteria have not been independently validated. Furthermore, in a study of 74 cases with simultaneously detected endometrial and ovarian carcinomas, only 8 % of ovarian tumours exhibited a micronodular configuration and only 19 % had a less than 5 cm diameter [25]. Bilateral involvement was established in only 13.6 % of patients with surgically proven ovarian metastases from clinical stage I endometrial carcinoma [62].
Assessment of the histological subtype and grade of synchronous ovarian and endometrial tumours is important in establishing the correct diagnosis. Independent primaries are most probably represented by low-grade endometrioid adenocarcinomas, notably in the presence of atypical endometrial hyperplasia or ovarian endometriosis [9, 115]. High histological grade of the endometrial tumour and bilateral ovarian malignancy, characteristically with surface involvement and a micronodular pattern, suggest a uterine primary with adnexal metastases. Contrary to previous belief, depth of myometrial invasion and presence of lymphovascular invasion (LVI) have not been substantiated as reliable discriminators, since 36–100 % of genetically proven metastatic tumours demonstrate less than 50 % of myometrial invasion and lack of LVI, implying a transtubal pathway of disease dissemination [115, 116]. Location of the uterine tumour on the periphery of the myometrium with relative sparing of the endometrium favours the diagnosis of an ovarian primary with uterine infiltration [9]. A serous histology of synchronous endometrial and ovarian tumours poses similar diagnostic considerations, since uterine serous carcinoma demonstrates a marked tendency for extrauterine spread, mainly to the ovaries and peritoneum, even when the tumour is confined to an endometrial polyp without myometrial invasion, and conversely ovarian serous carcinoma may metastasise to the uterus [9]. In problematic cases immunohistochemistry may be helpful, as 95–97 % of ovarian serous carcinomas display positive reactivity to Wilms’ tumour susceptibility gene 1 (WT1) (68 % with a moderate to strong positivity in >50 % of tumour cells), whereas 80–100 % of uterine serous carcinomas are negative [117, 118]. Furthermore, molecular profiling has been pursued with DNA flow cytometry, loss of chromosomal heterozygosity, microsatellite instability and analyses of alterations in PTEN and β-catenin, in order to determine the mono- or polyclonality of concomitant endometrial and ovarian tumours [109, 115, 116, 119–122].
On imaging, metastases from uterine cancer tend to be bilateral complex masses of smaller size and higher vascularity than their nongenital counterparts [64]. A solid composition has been reported in 85 % and a mixed multilocular/solid in 15 % of metastases [64]. Occasionally the synchronous primary endometrial tumour may be depicted on cross-sectional imaging and thus raise suspicion for the metastatic nature of the ovarian lesions (Figs. 6 and 7). However, endometrial thickening is present in 20–35 % of primary endometrioid ovarian carcinoma, which, nevertheless, appears as a large unilateral mass in 75 % of cases [123] (Fig. 8).
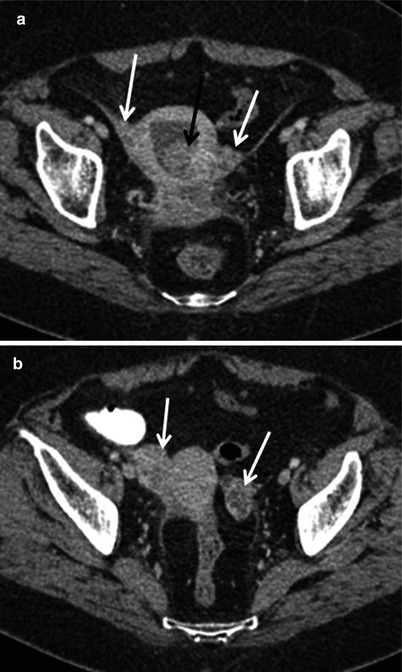
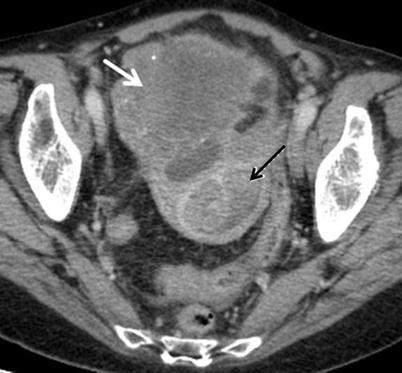
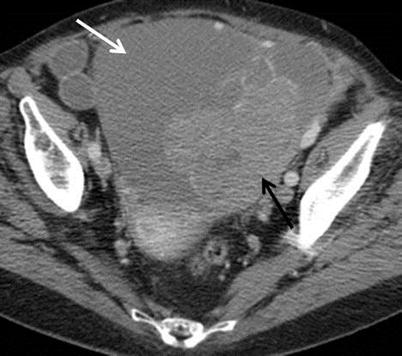

Fig. 6
Adnexal tumours in a 61-year-old patient with synchronous endometrial cancer. Axial contrast-enhanced CT images (a, b) demonstrate bilateral, small, complex ovarian lesions with heterogeneous enhancement (white arrows). A lobulated, enhancing solid tumour is depicted within the endometrial cavity (black arrow in a). A grade 3 endometrioid endometrial cancer was diagnosed on histology. High-grade endometrial tumour with bilateral small ovarian lesions is highly suggestive of secondary ovarian deposits

Fig. 7
Adnexal tumour in a 71-year-old patient with concomitant endometrial cancer. Axial contrast-enhanced CT image demonstrates a bulky, heterogeneously enhancing, partly cystic/necrotic complex right ovarian mass (white arrow) with minimal foci of calcification and a solid tumour expanding the endometrial cavity (black arrow). Histology revealed a grade 3 endometrioid adenocarcinoma of endometrium. In the context of endometrial cancer, the complex adnexal mass is likely to be a metastatic deposit in the right ovary

Fig. 8
Primary endometrioid adenocarcinoma of the ovary in an 80-year-old woman who presented with vaginal bleeding. Axial contrast-enhanced CT image demonstrates a unilateral left, bulky, complex adnexal mass with a dominant cystic portion (white arrow) and a large, solid, lobulated mural component arising from its lateral wall (black arrow). Histology revealed a primary grade 2 endometrioid ovarian adenocarcinoma. The large size and unilaterality of the lesion would be uncommon for metastases, but otherwise the imaging features are non-specific
Metastases from Breast Primary
Breast origin accounts for 31–38 % of ovarian metastases [19, 42]. In patients with breast cancer, the estimated lifetime risk for developing ovarian cancer is approximately twofold, increasing to threefold for women younger than 40 years and to sevenfold in the presence of a family history of breast or ovarian cancer [124]. In a 15-year follow-up study of 644 patients with stage I and II breast cancer, 55 (9 %) were diagnosed with a subsequent nonmammary malignancy, among whom the most frequent primary type (20 %) was ovarian [125]. The frequent association between breast and ovarian cancer has been ascribed to genetic predisposition; mutations in BRCA1 and BRCA2 genes, which mediate the homologous recombination DNA repair pathway, have been found in 5–15 % of ovarian [126] and in 2–6 % of breast malignancies [127]. For breast cancer the average cumulative lifetime risk in BRCA1- and BRCA2-mutation carriers has been reported as 54–65 % and 23–45 %, respectively [128, 129], whereas for ovarian cancer the corresponding estimates are 39–50 % and 11–27 % [128, 130, 131]. Among women with a history of breast cancer of any stage who develop an adnexal lesion, 18–50 % have a malignant diagnosis; in this subset of patients, 27–51 % of lesions have been reported of metastatic rather than of primary origin [66, 132, 133]. The prevalence of ovarian metastases critically depends on primary disease stage, as in patients with clinical stage IV breast cancer 58–68 % of all discovered adnexal masses represent metastases, in contrast to 0 % in patients with stage I disease [66, 134]. Typically the diagnosis of the primary mammary tumour precedes metastatic manifestations in 97–98 % of cases, with a median interval related to clinical stage, ranging between 0 months for stage IV and 24 months for stage II [2, 42]. By the time of diagnosis of ovarian involvement, most patients (73 %) have overt extra-ovarian metastatic disease [42].
The infiltrating lobular type of breast cancer is more likely to metastasise to the ovary; in an autopsy series of 92 metastatic breast cancers, 36 % of ovarian metastases originated from a lobular histology compared to 2.6 % from ductal (p < 0.002) [135]. However, due to the highest incidence of the infiltrating ductal subtype among breast cancers [8], 55–73 % of breast-derived ovarian metastases are from ductal histology [136, 137]. In contrast to metastases from other organs, the diagnosis of breast cancer typically (86–97 %) precedes that of the ovarian mass with an overall median time of 11–97 months [2, 42, 138].
Typically ovarian metastases from breast cancer feature small (<5 cm) tumour size and bilaterality (85 and 64 %, respectively) [42]. On imaging they mostly manifest as solid tumours with a multinodular pattern and a minor cystic component [80] (Figs. 9 and 10). Normal gross appearances have been reported in 46 % of involved ovaries with 31 % having only microscopic (<1 mm) tumour foci [42], thus highlighting the difficulty of detection on imaging. The majority (97 %) of macroscopically enlarged tumours display a diffusely solid or multinodular pattern, whereas a predominantly cystic appearance or necrosis involving >10 % of tumour have been reported in only 3 and 10 % of cases, respectively [42].
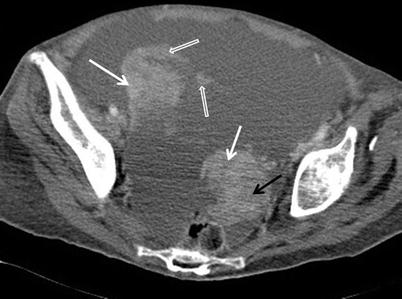
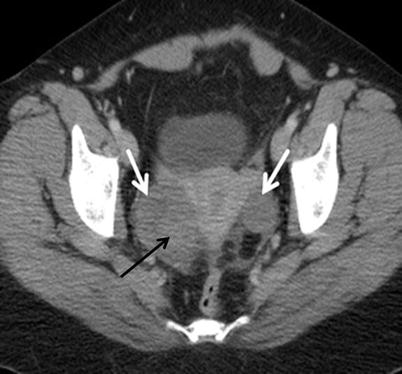

Fig. 9
Metachronous metastatic adnexal tumours in a 51-year-old woman with a 13-month history of mastectomy for stage IIIA infiltrating ductal carcinoma of the breast. The patient presented with abdominal distension and had local recurrence in the anterior thoracic wall. Axial contrast-enhanced CT demonstrates bilateral complex ovarian masses, with a prevailing solid component with avid enhancement (white arrows) and a minor cystic component (black arrow). The ill-defined shape of the lesions is atypical for breast metastases. There is marked ascites and scattered peritoneal nodularity (open arrows), suggestive of peritoneal dissemination

Fig. 10
Metachronous ovarian metastases in a 39-year-old woman with history of grade II invasive ductal breast carcinoma. Interval time from primary to metastatic diagnosis was 65 months. Axial contrast-enhanced CT image demonstrates relatively small (<5 cm) bilateral complex solid/cystic adnexal masses (white arrows) with preservation of the ovarian contour and moderate enhancement of the solid components (black arrow). The patient also had pulmonary and osseous secondary deposits
The microscopic histopathological features of metastases with a predominantly glandular or papillary pattern may be evocative of primary epithelial tumours [139]. Immuno-staining against oestrogen and progesterone receptors (ER and PR) is commonly positive for both primary and metastatic tumours. Nevertheless, intense and diffuse reactivity towards WT1 and carcinoembryonic antigen 125 (CA125) is displayed in 76–78 % and 78–90 % of primary ovarian cancers, respectively, compared to 0–3 % and 12–40 % of metastases from breast cancer, thus highlighting the potential of immunohistochemical panels for correct diagnosis [140, 141].
Lymphoma
Ovarian lymphoma is most commonly encountered in the presence of systemic disease rather than as a primary tumour and is almost exclusively of the non-Hodgkin type (particularly diffuse large B cell and follicular lymphoma) [142]. On CT the lesions are typically of large size (mean diameter 7.6–12 cm), bilateral in approximately 60 % of cases, well-defined and homogeneously hypodense, lacking calcification and displaying mild to moderate contrast enhancement [64, 142, 143] (Fig. 11). Areas of necrosis and haemorrhage are rare despite the frequent large dimensions of lesions. On MRI lesions demonstrate homogeneously low T1 and intermediate to high T2 signal intensity, which is typically lower than ovarian carcinoma [142, 143]. Another reported feature is preservation of ovarian shape and presence of T2-hyperintense septa with marked contrast enhancement [144]. Concomitant imaging findings, such as widespread lymphadenopathy, hepatosplenomegaly and bone marrow involvement, may aid towards the diagnosis of a haematological malignancy.
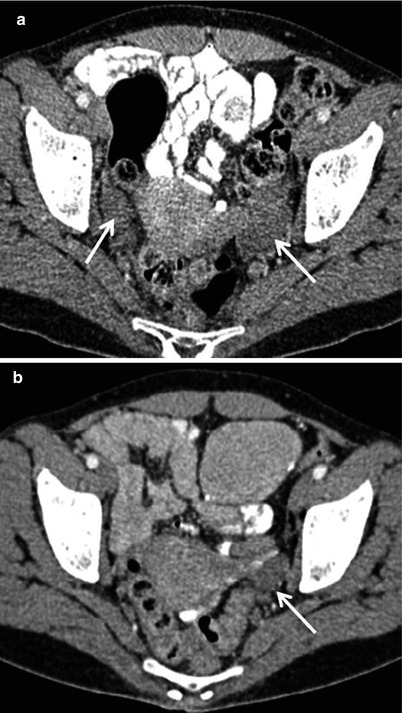

Fig. 11
Adnexal metastases in a 63-year-old patient with a history of diffuse large B cell non-Hodgkin lymphoma. Axial contrast-enhanced CT image (a) demonstrates bilateral, homogeneously hypodense, minimally enhancing ovarian lesions with smooth margins and preserved ovarian contour (arrows). A post-treatment contrast-enhanced CT image (b) demonstrates resolution of the right-sided tumour and a significant reduction in size of the left (arrow)
Malignant Melanoma
Most ovarian melanomas arise from a cutaneous or rarely choroidal tumour, although primary melanomas have been reported associated with teratomas [145, 146]. A definite history of prior melanoma is available in over 60 % of cases, but the interval between primary diagnosis and ovarian metastasis may be long, ranging from 15 to 228 months (mean 77.7 months) [146]. Extensive extra-ovarian metastatic disease is present in approximately 50–90 % at the time of presentation. Macroscopic melanin pigmentation, indicating the diagnosis, is present in only 22–35 % of tumours and often only focally [145–147]. Moreover, a similar brown colourisation may be produced by the lipochrome pigment of a steroid cell tumour, whereas amelanotic melanoma may be mistaken for a lipid-poor steroid cell tumour [8]. Average tumour size is approximately 10 cm (range 4.5–23 cm) and unilaterality is reported in 60–82 % [146, 148]. A minor or dominant cystic component is found in approximately 80–90 % of tumours [146] (Fig. 12). Imaging features are non-specific but when adequately present, melanin can be detected by its increased T1 signal intensity; differentiation from teratomas and endometriomas may be aided by the peripheral location of melanin-induced T1-hyperintensity in contrast to centrally located sebaceous/fatty and hemorrhagic components, respectively [149].
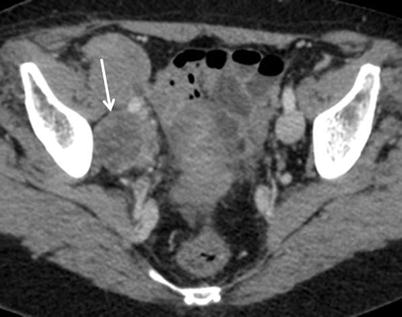

Fig. 12
Biopsy-proven adnexal metastasis in a 39-year-old patient with a history of cutaneous malignant melanoma excised 16 months previously. Axial contrast-enhanced CT image shows non-specific features of a unilateral, predominantly cystic right ovarian lesion (arrow), with sparse solid enhancing components
Conclusion
The ovaries are metastatic hosts to a multitude of primary tumours. The extensive overlap of imaging properties between primary and secondary ovarian tumours precludes a reliable distinction on the basis of CT/MRI-derived criteria. However, in conjunction with the clinical history, recognition of certain typical patterns of ovarian metastases depending on primary disease may facilitate the diagnosis. The principal imaging features and diagnostic considerations according to main metastatic tumour types are summarised in Table 1. A high suspicion index for secondary ovarian involvement is warranted in the setting of a known primary malignancy, especially of gastrointestinal, haematologic and melanoma origin. In approximately 40–50 % of cases, mainly of gastric and colorectal cancer, in which the discovery of adnexal disease precedes the primary diagnosis, features such as bilaterality, a predominantly solid appearance and lack of multilocularity may alert the radiologist to the possibility of a Krukenberg tumour, so that further diagnostic work-up be pursued. A prevalent cystic appearance, combined with bilateral involvement and relatively small tumour size, may be indicative of metastatic mucinous tumour, particularly of gastrointestinal source. Bilaterality and small tumour size in a patient with a history of advanced breast cancer raise suspicion for metastases. In synchronous endometrial and ovarian tumours distinction on imaging is increasingly challenging, although bilaterality and solid composition may suggest a metastatic relationship.
Table 1
Imaging features and diagnostic considerations according to metastatic tumour types
Metastatic tumour type | Imaging feature | Diagnostic considerations | |||
|---|---|---|---|---|---|
Laterality | Size | Composition | MR signal intensity | ||
Krukenberg | T2-hypointensity in solid components due to collagen/fibrosis | Needs to be distinguished from other T2-hypointense tumours (fibroma, fibrothecoma), usually unilateral | |||
Mucinous (non-Krukenberg) | Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||||